Anti-Inflammatory Effects of Morinda citrifolia Extract against Lipopolysaccharide-Induced Inflammation in RAW264 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents
2.3. Cell Culture System
2.4. RNA Isolation and Real-Time RT-PCR Analysis
2.5. Statistical Analyses
3. Results
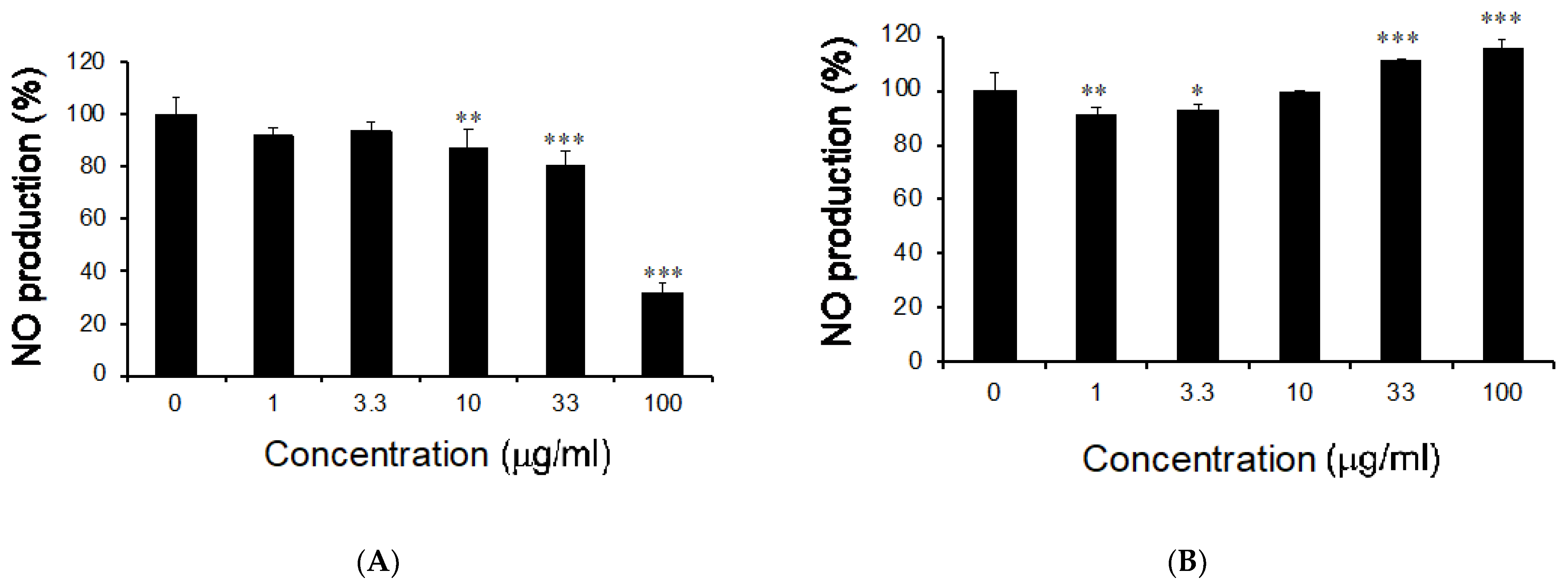
3.1. Effects of M. citrifolia Seeds Extract on Nitric Oxide Production
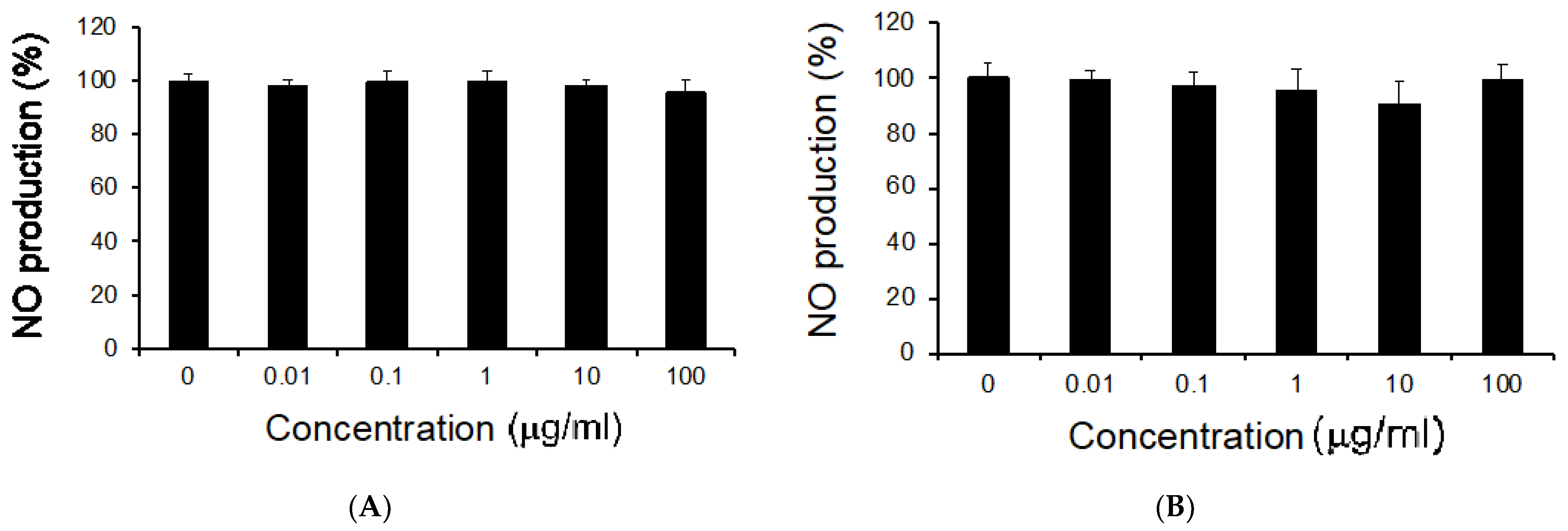
3.2. Effects of M. citrifolia Seeds Extract on Cell Viability
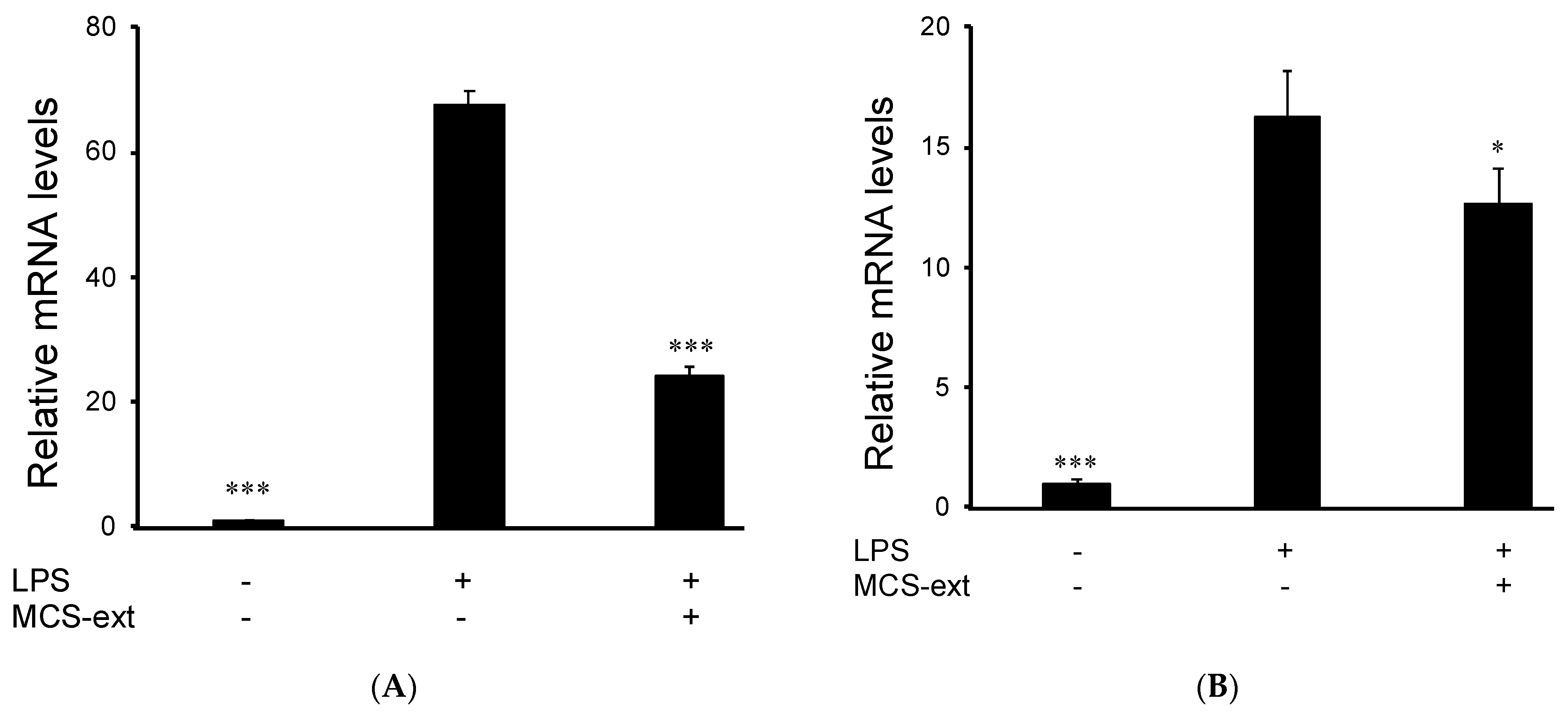
3.3. Effects of M. citrifolia Seeds Extract on LPS-Induced Expression of Inflammatory Mediator
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anogeianaki, A.; Angelucci, D.; Cianchetti, E.; D’alessandro, M.; Maccauro, G.; Saggini, A.; Salini, V.; Caraffa, A.; Teté, S.; Conti, F.; et al. Atherosclerosis: A Classic Inflammatory Disease. Int. J. Immunopathol. Pharmacol. 2011, 24, 817–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Furuichi, K.; Wada, T.; Kaneko, S. Involvement of Inflammation in Autoinflammation and Autoimmune Disease. Inflamm. Regen. 2011, 31, 81–87. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in Inflammation, Repair and Regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of Cytokines and Nitric Oxide Involvement in Immuno-Pathogenesis of Inflammatory Bowel Diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-Derived Immunomodulators: An Insight on Their Preclinical Evaluation and Clinical Trials. Front. Plant Sci. 2015, 6, 655. [Google Scholar] [CrossRef]
- Mian-Ying, W.; West, B.J.; Jensen, C.J.; Nowicki, D.; Chen, S.; Palu, A.K.; Anderson, G. Morinda citrifolia (Noni): A Literature Review and Recent Advances in Noni Research. Acta Pharmacol. Sin. 2002, 23, 1127–1141. [Google Scholar]
- Li, R.W.; Myers, S.P.; Leach, D.N.; Lin, G.D.; Leach, G. A Cross-Cultural Study: Anti-Inflammatory Activity of Australian and Chinese Plants. J. Ethnopharmacol. 2003, 85, 25–32. [Google Scholar] [CrossRef]
- Zaidan, M.R.S.; Noor Rain, A.; Badrul, A.R.; Adlin, A.; Norazah, A.; Zakiah, I. In Vitro Screening of Five Local Medicinal Plants for Antibacterial Activity Using Disc Diffusion Method. Trop. Biomed. 2005, 22, 165–170. [Google Scholar] [PubMed]
- Brown, A.C. Anticancer activity of Morinda citrifolia (Noni) fruit: A review: Anticancer activity of Morinda citrifolia (noni) fruit. Phytother. Res. 2012, 26, 1427–1440. [Google Scholar] [CrossRef]
- Dussossoy, E.; Brat, P.; Bony, E.; Boudard, F.; Poucheret, P.; Mertz, C.; Giaimis, J.; Michel, A. Characterization, Anti-Oxidative and Anti-Inflammatory Effects of Costa Rican Noni Juice (Morinda citrifolia L.). J. Ethnopharmacol. 2011, 133, 108–115. [Google Scholar] [CrossRef]
- Abou Assi, R.; Darwis, Y.; Abdulbaqi, I.M.; Khan, A.A.; Vuanghao, L.; Laghari, M.H. Morinda citrifolia (Noni): A Comprehensive Review on Its Industrial Uses, Pharmacological Activities, and Clinical Trials. Arab. J. Chem. 2017, 10, 691–707. [Google Scholar] [CrossRef]
- Almeida, É.S.; Oliveira, D.; Hotza, D. Properties and Applications of Morinda citrifolia (Noni): A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef] [Green Version]
- Saraphanchotiwitthaya, A.; Sripalakit, P. Anti-Inflammatory Effect of Morinda citrifolia Leaf Extract on Macrophage RAW 264.7 Cells. ScienceAsia 2015, 41, 5. [Google Scholar] [CrossRef] [Green Version]
- Ralph, P.; Nakoinz, I. Antibody-Dependent Killing of Erythrocyte and Tumor Targets by Macrophage-Related Cell Lines: Enhancement by PPD and LPS. J. Immunol. 1977, 119, 950–954. [Google Scholar] [PubMed]
- West, B.J.; Deng, S.; Palu, A.K.; Jensen, C.J. Morinda citrifolia Linn. (Rubiaceae) Leaf Extracts Mitigate UVB-Induced Erythema. J. Nat. Med. 2009, 63, 351–354. [Google Scholar] [CrossRef]
- West, B.J.; Deng, S.; Jensen, C.J. Nutrient and Phytochemical Analyses of Processed Noni Puree. Food Res. Int. 2011, 44, 2295–2301. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of Nitric Oxide in Inflammatory Diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Alley, E.W.; Murphy, W.J.; Russell, S.W. A Classical Enhancer Element Responsive to Both Lipopolysaccharide and Interferon-Gamma Augments Induction of the INOS Gene in Mouse Macrophages. Gene 1995, 158, 247–251. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Dussossoy, E.; Bony, E.; Michel, A.; Brat, P.; Vaillant, F.; Boudard, F.; Giaimis, J. Anti-oxidative and anti-inflammatory effects of the Morinda citrifolia Fruit (NONI). Acta Hortic. 2014, 69–73. [Google Scholar] [CrossRef]
- Dussossoy, E.; Bichon, F.; Bony, E.; Portet, K.; Brat, P.; Vaillant, F.; Michel, A.; Poucheret, P. Pulmonary Anti-Inflammatory Effects and Spasmolytic Properties of Costa Rican Noni Juice (Morinda citrifolia L.). J. Ethnopharmacol. 2016, 192, 264–272. [Google Scholar] [CrossRef]
- Mohamad Shalan, N.A.A.; Mustapha, N.M.; Mohamed, S. Morinda citrifolia Leaf Enhanced Performance by Improving Angiogenesis, Mitochondrial Biogenesis, Antioxidant, Anti-Inflammatory & Stress Responses. Food Chem. 2016, 212, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J. TNF-Mediated Inflammatory Disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Cerami, A. The Biology of Cachectin/TNF—A Primary Mediator of the Host Response. Annu. Rev. Immunol. 1989, 7, 625–655. [Google Scholar] [CrossRef] [PubMed]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the Role of TNF-α and IFN-γ Activation on the Dynamics of INOS Gene Expression in LPS Stimulated Macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Yu, J.S.; Huang, P.; Qader, M.; Manavalan, A.; Wu, X.; Kim, J.C.; Pang, C.; Cao, S.; Kang, K.S.; et al. Identification of Anti-Inflammatory Compounds from Hawaiian Noni (Morinda citrifolia L.) Fruit Juice. Molecules 2020, 25, 4968. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanikawa, T.; Kitamura, M.; Hayashi, Y.; Tomida, N.; Uwaya, A.; Isami, F.; Inoue, Y. Anti-Inflammatory Effects of Morinda citrifolia Extract against Lipopolysaccharide-Induced Inflammation in RAW264 Cells. Medicines 2021, 8, 43. https://doi.org/10.3390/medicines8080043
Tanikawa T, Kitamura M, Hayashi Y, Tomida N, Uwaya A, Isami F, Inoue Y. Anti-Inflammatory Effects of Morinda citrifolia Extract against Lipopolysaccharide-Induced Inflammation in RAW264 Cells. Medicines. 2021; 8(8):43. https://doi.org/10.3390/medicines8080043
Chicago/Turabian StyleTanikawa, Takashi, Masashi Kitamura, Yasuhiro Hayashi, Natsumi Tomida, Akemi Uwaya, Fumiyuki Isami, and Yutaka Inoue. 2021. "Anti-Inflammatory Effects of Morinda citrifolia Extract against Lipopolysaccharide-Induced Inflammation in RAW264 Cells" Medicines 8, no. 8: 43. https://doi.org/10.3390/medicines8080043
APA StyleTanikawa, T., Kitamura, M., Hayashi, Y., Tomida, N., Uwaya, A., Isami, F., & Inoue, Y. (2021). Anti-Inflammatory Effects of Morinda citrifolia Extract against Lipopolysaccharide-Induced Inflammation in RAW264 Cells. Medicines, 8(8), 43. https://doi.org/10.3390/medicines8080043





