Encephalic Leukocytoclastic Vasculitis during Treatment with Sunitinib for Renal Cell Carcinoma: A Case Report
Abstract
1. Introduction
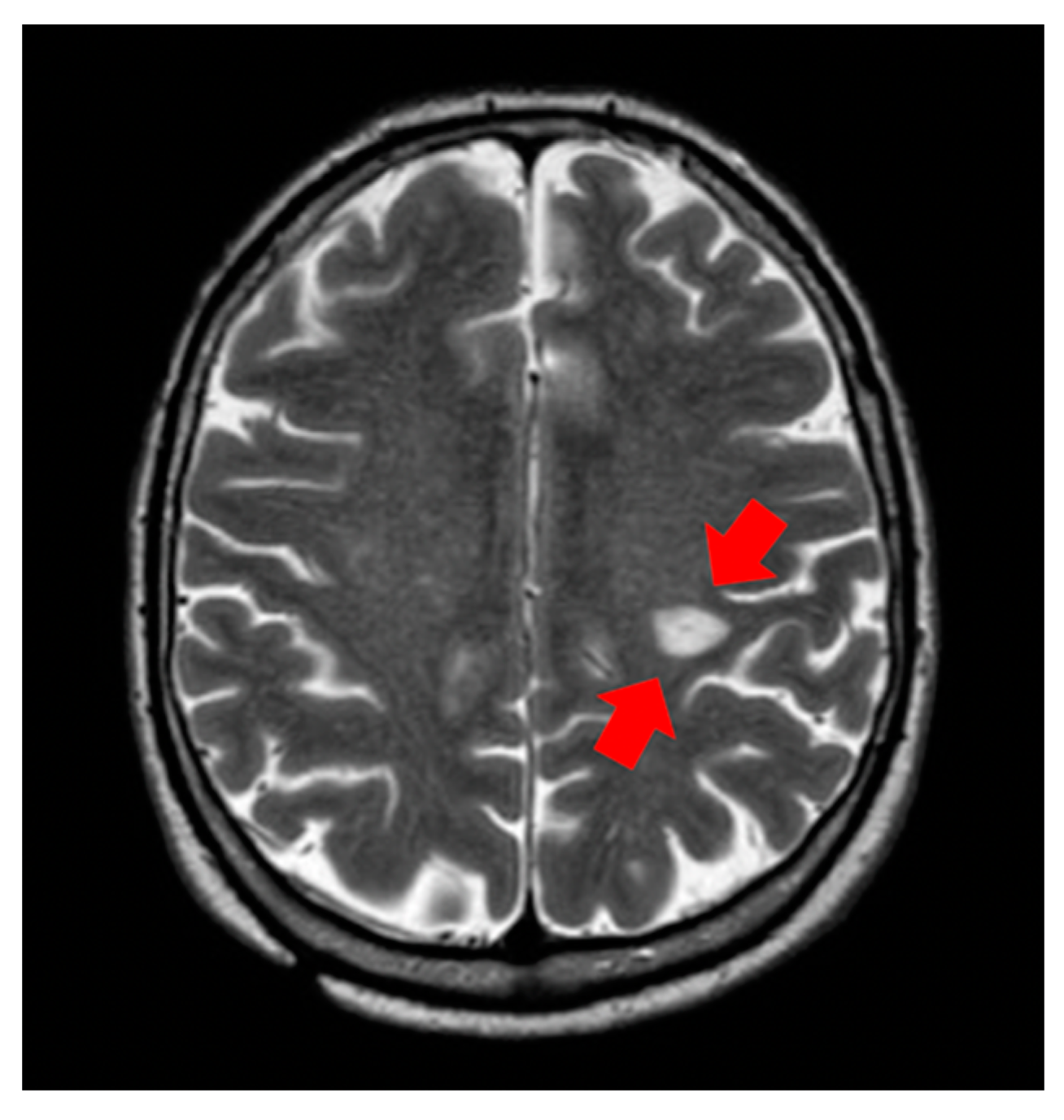
2. Cases and Methods
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ljunberg, B.; Campbell, S.C.; Cho, H.Y.; Jacqmin, D.; Lee, J.E.; Weikert, S.; Kiemeney, L.A. The epidemiology of renal cell carcinoma. Eur. Urol. 2011, 60, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grunwald, V.; Gillessen, S.; Horwich, A. ESMO Guidelines Committee. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Buti, S.; Conti, A.; Porta, C.; Procopio, G.; Sternberg, C.N.; Bracarda, S.; Basso, M.; De Giorgi, U.; Rizzo, M.; et al. Prognostic significance of host immune status in patients with late relapsing renal cell carcinoma treated with targeted therapy. Target Oncol. 2015, 10, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Bacik, J.; Murphy, B.A.; Russo, P.; Mazumdar, M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J. Clin. Oncol. 2002, 20, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Motzer, R.J. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2017, 376, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Mollica, V.; Rizzo, A.; Cosmai, L.; Rizzo, M.; Porta, C. Safety evaluation of immune-based combinations in patients with advanced renal cell carcinoma: A systematic review and meta-analysis. Expert Opin. Drug Saf. 2020, 19, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.; Drake, C.G. Kidney Cancer: An Overview of Current Therapeutic Approaches. Urol. Clin. North Am. 2020, 47, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Bhojani, N.; Jeldres, C.; Patard, J.J.; Perrotte, P.; Suardi, N.; Hutterer, G.; Patenaude, F.; Oudard, S.; Karakiewicz, P.P. Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur. Urol. 2008, 53, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Soria, J.C.; Spatz, A.; Le Cesne, A.; Malka, D.; Pautier, P.; Wechsler, J.; Lhomme, C.; Escudier, B.; Boige, V.; et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005, 6, 491–500. [Google Scholar] [CrossRef]
- Schmid, T.A.; Gore, M.E. Sunitinib in the treatment of metastatic renal cell carcinoma. Ther. Adv. Urol. 2016, 8, 348–371. [Google Scholar] [CrossRef] [PubMed]
- Palapattu, G.S.; Kristo, B.; Rajfer, J. Paraneoplastic syndromes in urologic malignancy: The many faces of renal cell carcinoma. Rev. Urol. 2002, 4, 163–170. [Google Scholar] [PubMed]
- Radic, M.; Martinovic Kaliterna, D.; Radic, J. Drug-induced vasculitis: A clinical and pathological review. Neth. J. Med. 2012, 70, 12–17. [Google Scholar] [PubMed]
- Fekete, G.L.; Fekete, L. Cutaneous leukocytoclastic vasculitis associated with erlotinib treatment: A case report and review of the literature. Exp. Ther. Med. 2019, 17, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Boeck, S.; Wollenberg, A.; Heinemann, V. Leukocytoclastic vasculitis during treatment with the oral EGFR tyrosine kinase inhibitor erlotinib. Ann. Oncol. 2007, 18, 1582–1583. [Google Scholar] [CrossRef] [PubMed]
- Kamo, H.; Shinozaki, E.; Sugase, T.; Mizunuma, N.; Taniguchi, S.; Gotoh, T.; Chin, L.; Tanaka, T.; Koga, L.; Yamaguchi, K. Leukocytoclastic vasculitis with purpura and renal failure induced by the anti-epidermal growth factor receptor antibody panitumumab: A case report. J. Med. Case Rep. 2019, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, T.; Tsubata, Y.; Hotta, T.; Hamaguchi, M.; Okuno, T.; Shiratsuki, Y.; Kodama, A.; Nakao, M.; Amano, Y.; Hamaguchi, S.; et al. Successful rechallenge with ceritinib after leukocytoclastic vasculitis during ceritinib treatment for non-small cell lung cancer harboring the EML4-ALK fusion protein. Oncotarget 2018, 9, 20213–20218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karadimou, A.; Migou, M.; Economidi, A.; Stratigos, A.; Kittas, C.; Dimopoulos, M.A.; Bamias, A. Leukocytoclastic vasculitis after long-term treatment with sunitinib: A case report. Case Rep. Oncol. 2011, 4, 385–391. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massucci, M.; Mollica, V.; Rizzo, A.; Ventrella, L.; Maggio, I.; Manuzzi, L.; Gatto, L.; Brandi, G.; Massari, F. Encephalic Leukocytoclastic Vasculitis during Treatment with Sunitinib for Renal Cell Carcinoma: A Case Report. Medicines 2021, 8, 5. https://doi.org/10.3390/medicines8010005
Massucci M, Mollica V, Rizzo A, Ventrella L, Maggio I, Manuzzi L, Gatto L, Brandi G, Massari F. Encephalic Leukocytoclastic Vasculitis during Treatment with Sunitinib for Renal Cell Carcinoma: A Case Report. Medicines. 2021; 8(1):5. https://doi.org/10.3390/medicines8010005
Chicago/Turabian StyleMassucci, Maria, Veronica Mollica, Alessandro Rizzo, Laura Ventrella, Ilaria Maggio, Lisa Manuzzi, Lidia Gatto, Giovanni Brandi, and Francesco Massari. 2021. "Encephalic Leukocytoclastic Vasculitis during Treatment with Sunitinib for Renal Cell Carcinoma: A Case Report" Medicines 8, no. 1: 5. https://doi.org/10.3390/medicines8010005
APA StyleMassucci, M., Mollica, V., Rizzo, A., Ventrella, L., Maggio, I., Manuzzi, L., Gatto, L., Brandi, G., & Massari, F. (2021). Encephalic Leukocytoclastic Vasculitis during Treatment with Sunitinib for Renal Cell Carcinoma: A Case Report. Medicines, 8(1), 5. https://doi.org/10.3390/medicines8010005






