Arctium lappa Extract Suppresses Inflammation and Inhibits Melanoma Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydroalchoolic Arctium lappa L. Extract
2.2. Animals
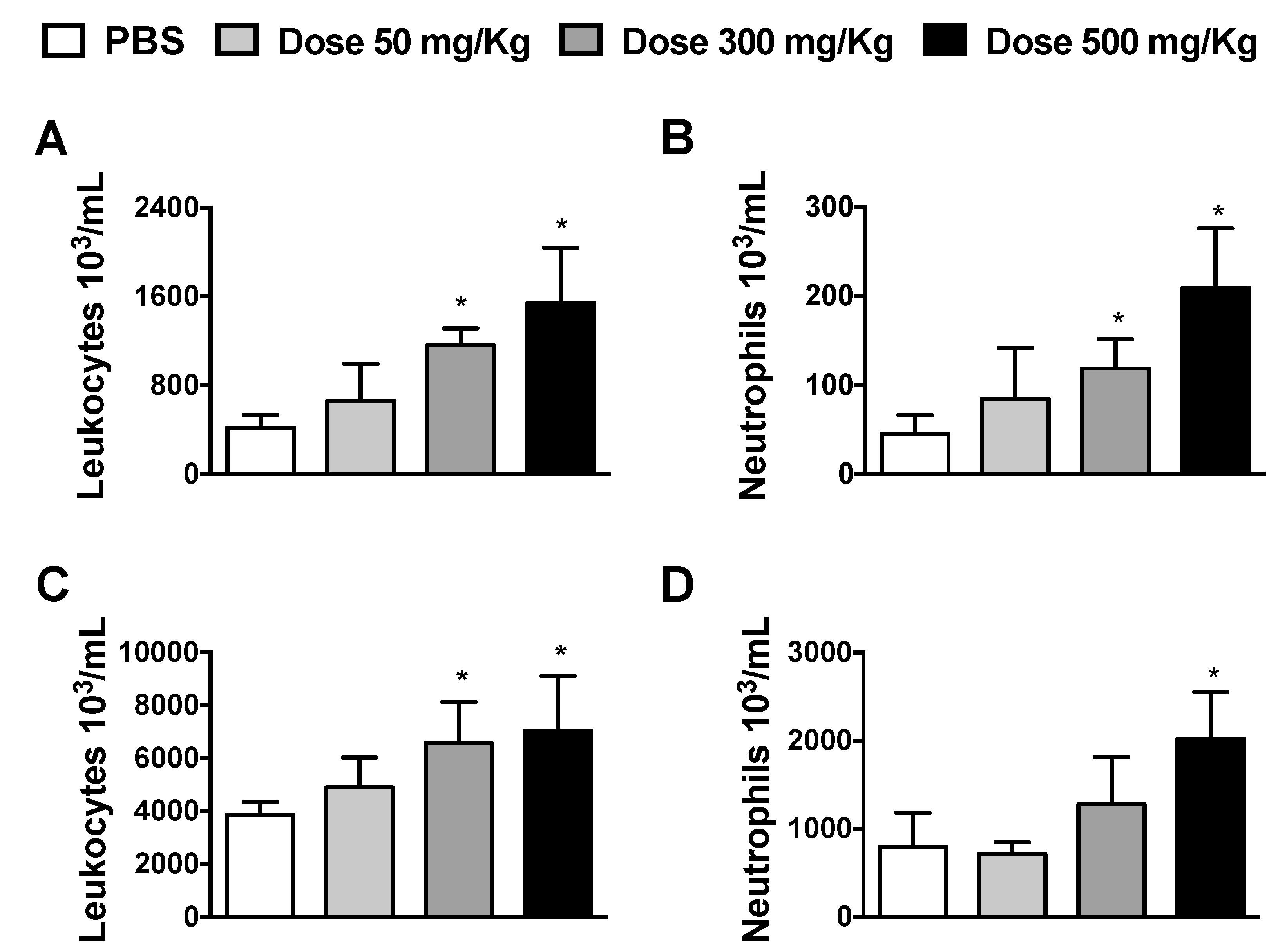
2.3. Determination of Alhe Dose and Leukocyte Count in Blood and the Peritoneal Cavity
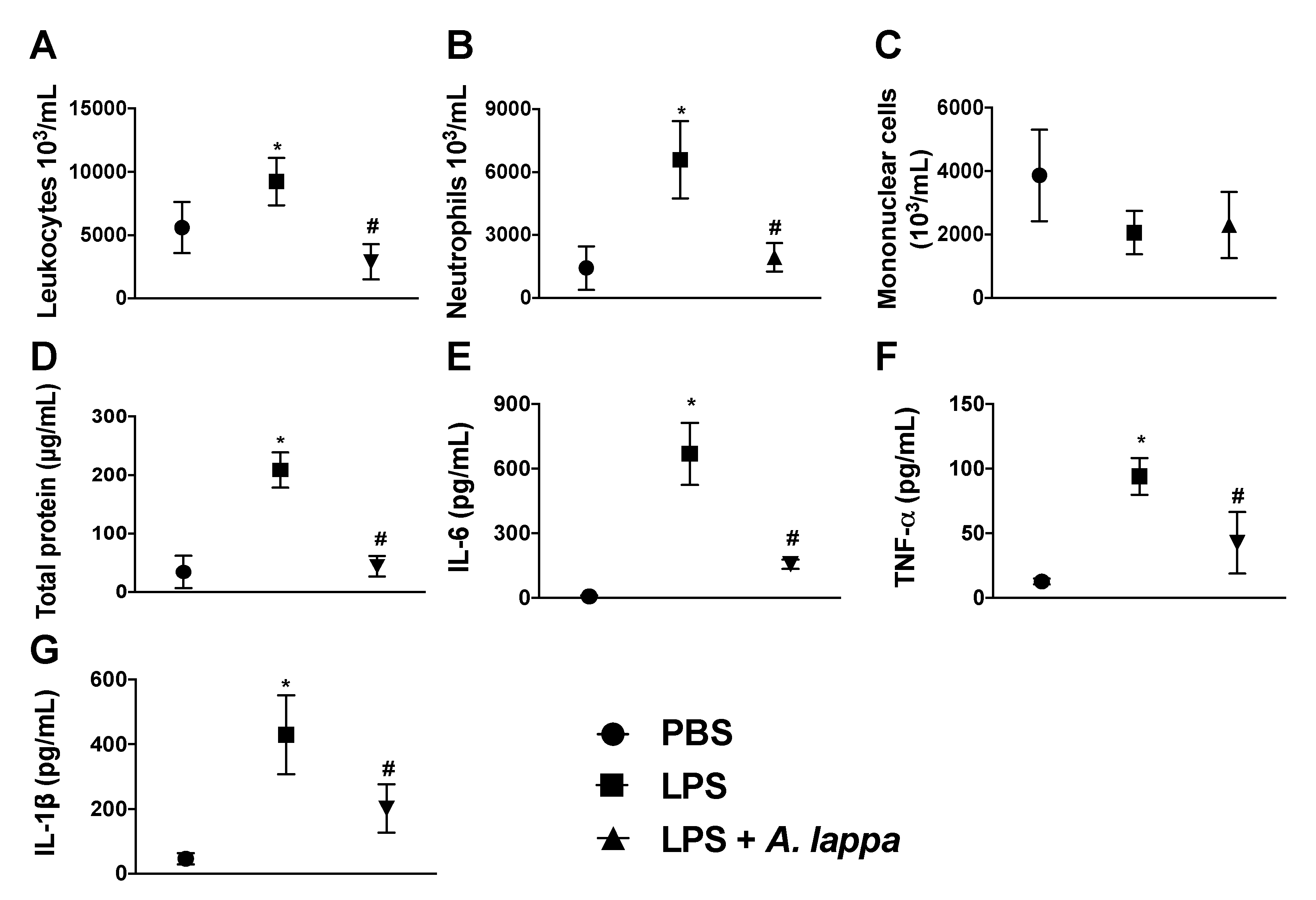
2.4. Air Pouch Induction and Stimuli Injection
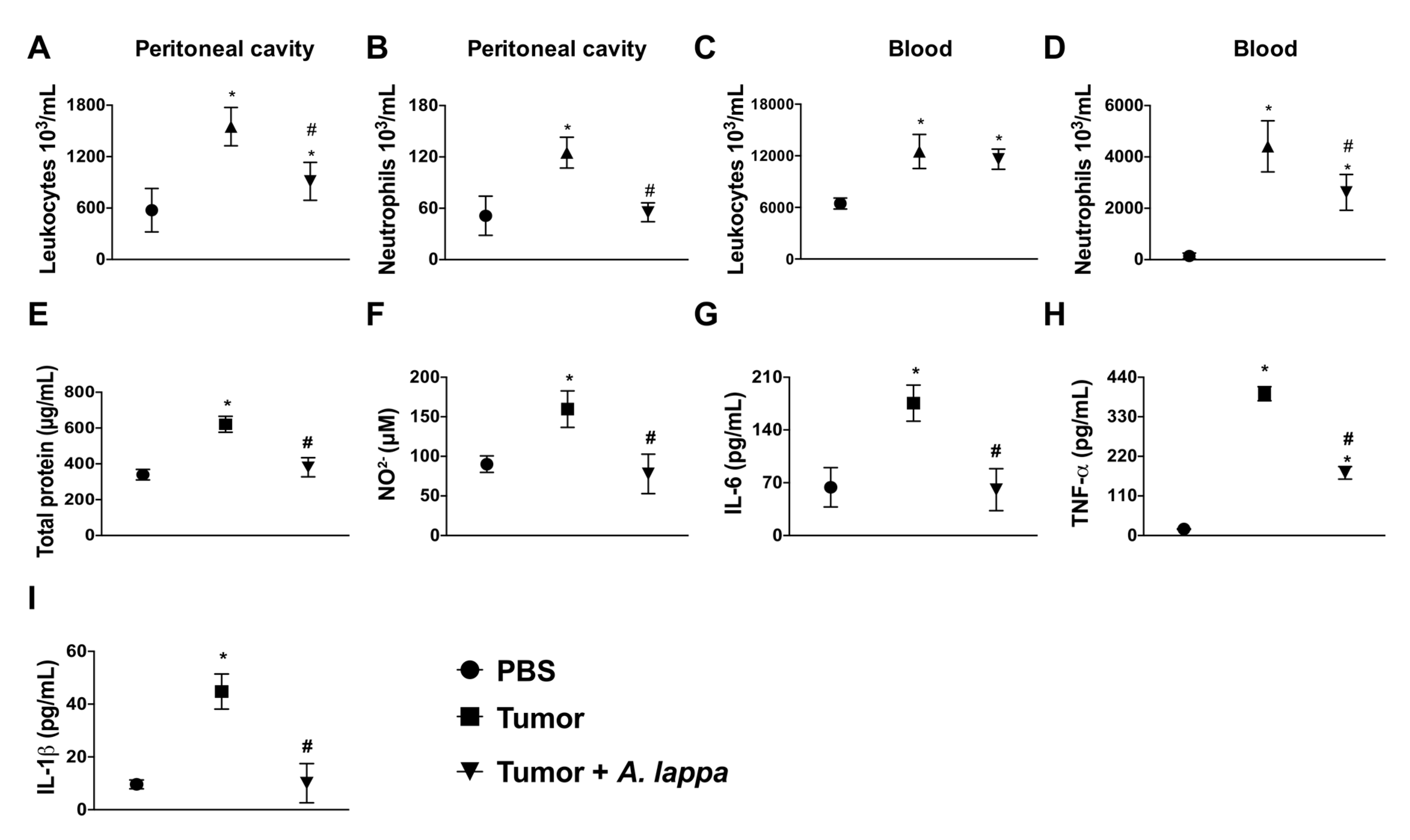
2.5. Anti-Tumoral Assay
2.6. Quantification of Nitric Oxide (NO) and Total Protein
2.7. Cytokine Quantification
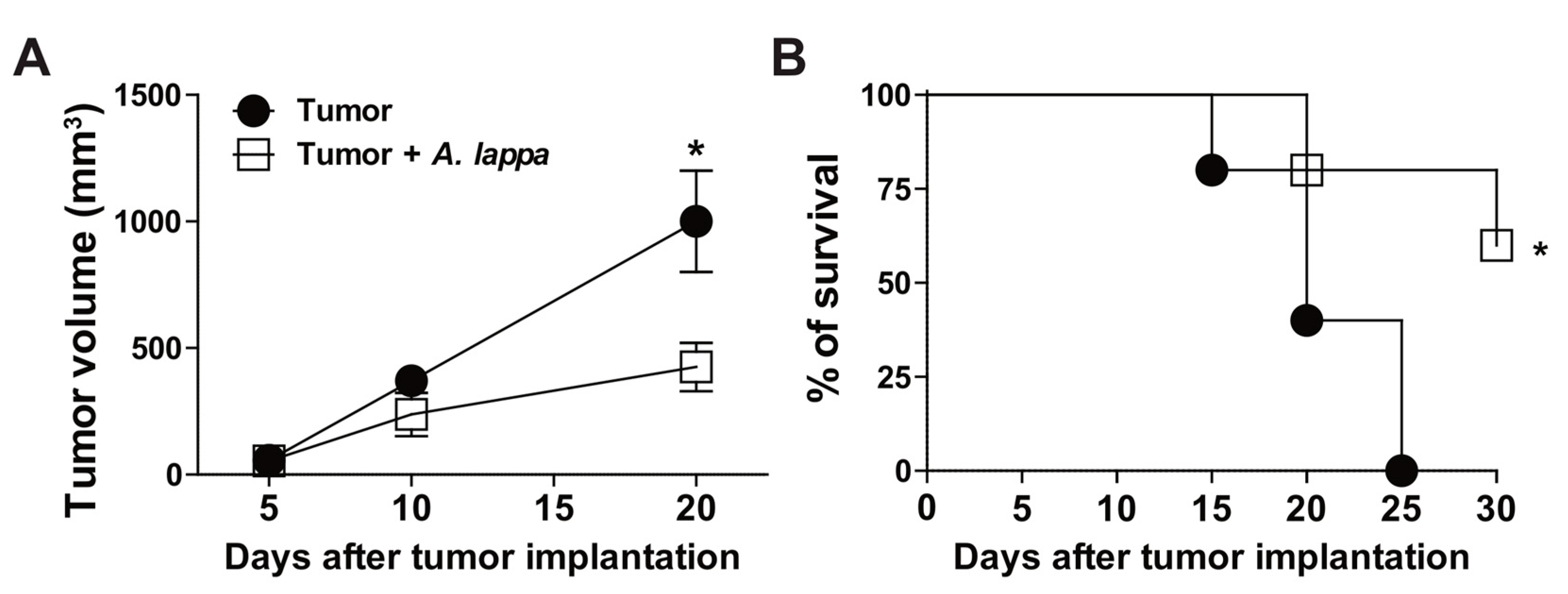
2.8. Tumor Evaluation
2.9. Statistical Analyses
3. Results
3.1. Dose-Dependent Effects of Alhe on Local and Peripheral Leukocyte Accumulation
3.2. Alhe Inhibits Leukocyte Recruitment and Activation in Response to LPS Challenge
3.3. Alhe Restricts Melanoma Progression and Mortality
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.J.; Kono, H.; Golenbock, D.; Reed, G.; Akira, S.; Rock, K.L. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 2007, 13, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The sterile inflammatory response. Annu. Rev. Immunol. 2010, 28, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Chinembiri, T.N.; du Plessis, L.H.; Gerber, M.; Hamman, J.H.; du Plessis, J. Review of natural compounds for potential skin cancer treatment. Molecules 2014, 19, 11679–11721. [Google Scholar] [CrossRef] [PubMed]
- Lippens, S.; Hoste, E.; Vandenabeele, P.; Agostinis, P.; Declercq, W. Cell death in the skin. Apoptosis 2009, 14, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Park, Y.; Subar, A.F.; Hollenbeck, A.R.; Leitzmann, M.F.; Schatzkin, A.; Abnet, C.C. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int. J. Cancer 2007, 121, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.L.; Elston, D.M.; Tyler, W.B.; Marks, V.J.; Ferringer, T. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol. Online J. 2010, 16, 4. [Google Scholar] [PubMed]
- Alifrangis, C.; Koizia, L.; Rozario, A.; Rodney, S.; Harrington, M.; Somerville, C.; Peplow, T.; Waxman, J. The experiences of cancer patients. QJM 2011, 104, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.; Parry, M.; Gill, P.; Mead, D.; Macbeth, F. Hard choices: A qualitative study of influences on the treatment decisions made by advanced lung cancer patients. Int. J. Palliat. Nurs. 2011, 17, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhao, L.; Zhong, R.; Peng, Y. The specific role of O(6)-methylguanine-DNA methyltransferase inhibitors in cancer chemotherapy. Future Med. Chem. 2018, 10, 1971–1996. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Fan, T.; Zhao, L.; Zhou, Y.; Zhong, R. The potential of combi-molecules with DNA-damaging function as anticancer agents. Future Med. Chem. 2017, 9, 403–435. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Fernández-Ortega, P.; Pud, D.; Ozden, G.; Scott, J.A.; Panteli, V.; Margulies, A.; Browall, M.; Magri, M.; Selvekerova, S.; et al. Use of complementary and alternative medicine in cancer patients: A European survey. Ann. Oncol. 2005, 16, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Sun, G.; Sun, X.; Zhao, L.; Zhong, R.; Peng, Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers 2019, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.C.; Haas, A.P.; von Poser, G.L.; Schapoval, E.E.; Elisabetsky, E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. J. Ethnopharmacol. 2004, 90, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.; Nakamura, C.V.; Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Memórias Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- De Melo, J.G.; Santos, A.G.; de Amorim, E.L.; do Nascimento, S.C.; de Albuquerque, U.P. Medicinal plants used as antitumor agents in Brazil: An ethnobotanical approach. Evid. Based Complement. Altern. Med. 2011, 2011, 365359. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.V.; Bergamo, D.C.; Pereira, J.O.; França Sde, C.; Pietro, R.C.; Silva-Sousa, Y.T. Antimicrobial activity of Arctium lappa constituents against microorganisms commonly found in endodontic infections. Braz. Dent. J. 2005, 16, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Predes Fde, S.; Diamante, M.A.; Foglio, M.A.; Dolder, H. Effects of Arctium lappa on Cadmium-Induced Damage to the Testis and Epididymis of Adult Wistar Rats. Biol. Trace Elem. Res. 2016, 173, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.; Leung, G.P.; Yu, P.H.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef]
- Lin, S.C.; Lin, C.H.; Lin, C.C.; Lin, Y.H.; Chen, C.F.; Chen, I.C.; Wang, L.Y. Hepatoprotective effects of Arctium lappa Linne on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. J. Biomed. Sci. 2002, 9, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Lu, J.; Kalauni, S.K.; Kurashima, Y.; Tezuka, Y.; Kadota, S.; Esumi, H. Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res. 2006, 66, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Hosono-Nishiyama, K.; Yamada, H. Antiproliferative and apoptotic effects of butyrolactone lignans from Arctium lappa on leukemic cells. Planta Med. 2006, 72, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, Y.; Dou, Y.; Ye, J.; Bian, D.; Wei, Z.; Tong, B.; Kong, L.; Xia, Y.; Dai, Y. Arctigenin but not arctiin acts as the major effective constituent of Arctium lappa L. fruit for attenuating colonic inflammatory response induced by dextran sulfate sodium in mice. Int. Immunopharmacol. 2014, 23, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Fronza, M.; Muhr, C.; da Silveira, D.S.; Sorgi, C.A.; Rodrigues, S.F.; Farsky, S.H.; Paula-Silva, F.W.; Merfort, I.; Faccioli, L.H. Hyaluronidase decreases neutrophils infiltration to the inflammatory site. Inflamm. Res. 2016, 65, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Ruiz de Luzuriaga, K.; Wagner, D.A.; Rand, W.; Istfan, N.; Young, V.R.; Tannenbaum, S.R. Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA 1981, 78, 7764–7768. [Google Scholar] [CrossRef] [PubMed]
- Looney, W.B.; Mayo, A.A.; Janners, M.Y.; Mellon, J.G.; Allen, P.; Salak, D.; Morris, H.P. Cell proliferation and tumor growth in hepatomas 3924A. Cancer Res. 1971, 31, 821–825. [Google Scholar] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef]
- Qu, X.; Tang, Y.; Hua, S. Immunological Approaches Towards Cancer and Inflammation: A Cross Talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, D.; Shi, X.; Liang, H.; Pang, Y.; Qin, N.; Chen, H.; Wang, J.; Yin, B.; Jiang, X.; et al. Transmembrane TNF-alpha mediates “forward” and “reverse” signaling, inducing cell death or survival via the NF-kappaB pathway in Raji Burkitt lymphoma cells. J. Leukoc. Biol. 2008, 84, 789–797. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Kim, J.Y.; Kim, K.W.; Park, M.K.; Moon, Y.; Kim, W.U.; Kim, H.Y. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res. Ther. 2004, 6, 120–128. [Google Scholar] [CrossRef]
- Tudan, C.; Jackson, J.K.; Blanis, L.; Pelech, S.L.; Burt, H.M. Inhibition of TNF-alpha-induced neutrophil apoptosis by crystals of calcium pyrophosphate dihydrate is mediated by the extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways up-stream of caspase 3. J. Immunol. 2000, 165, 5798–5806. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Souto, F.O.; Vieira, S.M.; Almeida, S.C.; Fukada, S.Y.; Xu, D.; Alves-Filho, J.C.; Cunha, T.M.; Guerrero, A.T.; Mattos-Guimaraes, R.B.; et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann. Rheum. Dis. 2010, 69, 1697–1703. [Google Scholar] [CrossRef]
- Zoccal, K.F.; Gardinassi, L.G.; Sorgi, C.A.; Meirelles, A.F.G.; Bordon, K.C.F.; Glezer, I.; Cupo, P.; Matsuno, A.K.; Bollela, V.R.; Arantes, E.C.; et al. CD36 Shunts Eicosanoid Metabolism to Repress CD14 Licensed Interleukin-1beta Release and Inflammation. Front. Immunol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Zoccal, K.F.; Sorgi, C.A.; Hori, J.I.; Paula-Silva, F.W.; Arantes, E.C.; Serezani, C.H.; Zamboni, D.S.; Faccioli, L.H. Opposing roles of LTB4 and PGE2 in regulating the inflammasome-dependent scorpion venom-induced mortality. Nat. Commun. 2016, 7, 10760. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, M.; Zuo, Z. Overview of the anti-inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacol. Sin. 2018, 39, 787–801. [Google Scholar] [CrossRef]
- He, Y.; Fan, Q.; Cai, T.; Huang, W.; Xie, X.; Wen, Y.; Shi, Z. Molecular mechanisms of the action of Arctigenin in cancer. Biomed. Pharmacother. 2018, 108, 403–407. [Google Scholar] [CrossRef]
- Ghafari, F.; Rajabi, M.R.; Mazoochi, T.; Taghizadeh, M.; Nikzad, H.; Atlasi, M.A.; Taherian, A. Comparing Apoptosis and Necrosis Effects of Arctium Lappa Root Extract and Doxorubicin on MCF7 and MDA-MB-231 Cell Lines. Asian Pac. J. Cancer Prev. 2017, 18, 795–802. [Google Scholar]
- Predes, F.S.; Ruiz, A.L.; Carvalho, J.E.; Foglio, M.A.; Dolder, H. Antioxidative and in vitro antiproliferative activity of Arctium lappa root extracts. BMC Complement. Altern. Med. 2011, 11, 25. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, Y.J.; Lu, Z.Z.; Li, B.B.; Sun, C.H.; Li, T.; Zhao, L.L.; Liu, Z.; Zhang, G.M.; Yao, J.C.; et al. Arctigenin Inhibits Liver Cancer Tumorigenesis by Inhibiting Gankyrin Expression via C/EBPalpha and PPARalpha. Front. Pharmacol. 2018, 9, 268. [Google Scholar] [CrossRef]
- Park, H.; Song, K.H.; Jung, P.M.; Kim, J.E.; Ro, H.; Kim, M.Y.; Ma, J.Y. Inhibitory Effect of Arctigenin from Fructus Arctii Extract on Melanin Synthesis via Repression of Tyrosinase Expression. Evid. Based Complement. Altern. Med. 2013, 2013, 965312. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Koyama, M.; Hitomi, T.; Yokota, T.; Kawanaka, M.; Nishikawa, A.; Germain, D.; Sakai, T. Arctiin induces cell growth inhibition through the down-regulation of cyclin D1 expression. Oncol. Rep. 2008, 19, 721–727. [Google Scholar] [CrossRef][Green Version]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Singel, K.L.; Segal, B.H. Neutrophils in the tumor microenvironment: Trying to heal the wound that cannot heal. Immunol. Rev. 2016, 273, 329–343. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, B.A.C.; Gardinassi, L.G.; Silveira, I.M.G.; Gallucci, M.G.; Tomé, M.A.; Oliveira, J.F.D.; Moreira, M.R.A.; Meirelles, A.F.G.; Faccioli, L.H.; Tefé-Silva, C.; et al. Arctium lappa Extract Suppresses Inflammation and Inhibits Melanoma Progression. Medicines 2019, 6, 81. https://doi.org/10.3390/medicines6030081
Nascimento BAC, Gardinassi LG, Silveira IMG, Gallucci MG, Tomé MA, Oliveira JFD, Moreira MRA, Meirelles AFG, Faccioli LH, Tefé-Silva C, et al. Arctium lappa Extract Suppresses Inflammation and Inhibits Melanoma Progression. Medicines. 2019; 6(3):81. https://doi.org/10.3390/medicines6030081
Chicago/Turabian StyleNascimento, Bruno A. C., Luiz G. Gardinassi, Inaê M. G. Silveira, Marília G. Gallucci, Mariana A. Tomé, Júlia Fernanda D. Oliveira, Mirella R. A. Moreira, Alyne F. G. Meirelles, Lúcia H. Faccioli, Cristiane Tefé-Silva, and et al. 2019. "Arctium lappa Extract Suppresses Inflammation and Inhibits Melanoma Progression" Medicines 6, no. 3: 81. https://doi.org/10.3390/medicines6030081
APA StyleNascimento, B. A. C., Gardinassi, L. G., Silveira, I. M. G., Gallucci, M. G., Tomé, M. A., Oliveira, J. F. D., Moreira, M. R. A., Meirelles, A. F. G., Faccioli, L. H., Tefé-Silva, C., & Zoccal, K. F. (2019). Arctium lappa Extract Suppresses Inflammation and Inhibits Melanoma Progression. Medicines, 6(3), 81. https://doi.org/10.3390/medicines6030081





