Abstract
Breast cancer is still one of the most prevalent cancers and a leading cause of cancer death worldwide. The key challenge with cancer treatment is the choice of the best therapeutic agents with the least possible toxicities on the patient. Recently, attention has been drawn to herbal compounds, in particular ginsenosides, extracted from the root of the Ginseng plant. In various studies, significant anti-cancer properties of ginsenosides have been reported in different cancers. The mode of action of ginsenoside Rg3 (Rg3) in in vitro and in vivo breast cancer models and its value as an anti-cancer treatment for breast cancer will be reviewed.
1. Metastatic Breast Cancer
Metastatic breast cancer (MBC) is classified as stage IV where the tumor has metastasized to distant organs such as bones, lungs, liver, or brain [1]. Breast tumors are subdivided into different categories based on the receptors expressed in the cells: estrogen receptor (ER)- or progesterone receptor (PR)- positive, human epidermal growth factor receptor 2 (HER2) positive, and triple negative breast tumors (ER-/PR-/HER2-) (Figure 1). Hormone receptor expressing tumors including luminal A or luminal B subtypes constitute the largest portion of patients and have the best prognosis, as these tumors are inherently less aggressive compared to other subtypes of breast cancer and that the majority of these tumors are responsive to hormone therapy options such as tamoxifen or letrozole [2]. HER2-expressing tumors constitute 15–20% of the patients [3]. The prognosis of this group of patients has improved following the introduction of targeted anti-HER2 medications, such as trastuzumab [4]. Some 15% of patients have triple negative (basal-like) breast cancer (TNBC) [5] and for these patients, chemotherapy remains the main treatment option [6]. TNBC is associated with younger age (<50 years) at diagnosis and BRCA1 mutation, and by the time of diagnosis have larger tumor size, higher grade tumor and the worst prognosis [7]. Recently, inhibitors of poly ADP ribose polymerase (PARP) have been found to be effective in the treatment of TNBC patients with BRCA-1 or -2 mutations. Likewise, platinum-based cytotoxic drugs are being tested in such patients [2]. However, still there is no ultimate cure for MBC and these patients suffer from the side effects of chemotherapy, until the tumor develops acquired resistance [8] and the patients succumb to the disease. Hence, many cancer researchers are actively looking for better treatment options to improve the quality of life of the MBC patients, reduce the toxicities of the chemotherapy regimens, restrict tumor metastasis, and improve survival. In this review, the medicinal herb ginseng and the important group of chemicals in its extract are suggested as one such therapy with the potential for reduced toxicity.
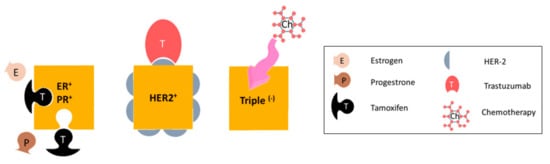
Figure 1.
Subtypes of metastatic breast cancer, based on receptor expression. Hormone-receptor expressing tumors are treated with anti-hormone therapy (such as tamoxifen), HER2-expressing tumors are given targeted anti-HER2 monoclonal antibody therapy such as trastuzimab. The main treatment option for triple negative breast tumors is chemotherapy.
2. Ginseng—History and Medicinal Use
Ginseng, with a long history of human use as a traditional medicine, has various pharmacological effects [9,10,11], and is widely used for its nutritional value as a food, energizing the body [10], improving body performance in sports, relieving menopausal symptoms, and alleviating sexual dysfunction [12]. Panax ginseng, also known as Chinese or Korean ginseng is a species of ginseng, the extract of which has the highest medicinal value among other species. Ginsenosides are the group of chemicals in this extract having the highest medicinal value [13]. So far, 38 ginsenosides have been identified [14]. Ginsenosides are saponins having a steroid-like hydrophobic backbone connected to sugar moieties. Based on their chemical structure, they are categorized into panaxadiol, panaxatriol, and oleanolic groups [13], with protopanaxadiols being the most abundant group. Protopanaxadiols include Rb1, Rb2, Rg3, Rh2, Rc, Rd, Ra, and F2 [12] and have various medicinal properties, including anti-diabetic effects, protection against cardiovascular diseases and anticancer properties [10,12].
Not all of these protopanaxadiols are available in ginseng extract. Commercial ginseng is produced either by air-drying or steaming (120°C, 4 h) the plant. These processes produce white or red ginseng, respectively [15,16,17]. Due to the conversions and chemical changes following heating, the heat processed or red ginseng has higher medicinal properties than white ginseng. Within this heating process, polar ginsenosides such as Rb1 or Rb3 convert to less polar ginsenosides such as Rg2 and Rg3 [15,18].
Ginsenoside Rg3 is one of the well-studied members of protopanaxadiols, and arises following loss of the sugar moiety on C20 in the heating process. Like other ginsenosides, Rg3 has a stereocenter on C20, giving it two epimers; 20(R)- and 20(S)-ginsenoside Rg3 (Figure 2). This is due to the selective attachment of the hydroxyl group to the C20 after losing the sugar structure. In the 20(R)-Rg3 epimer, the hydroxyl on C20 is far from the hydroxyl on C12, whilst in 20(S)-Rg3, these two hydroxyls are close to each other. Also, the alkene chain connected to C20 in 20(R)-Rg3 is more flexible compared to its fixed orientation in 20(S)-Rg3. This makes the alkene in 20(S)-Rg3 less accessible to water and more prone to hydrophobic interactions while leaving the hydroxyl groups to interact with the receptors. This may play a part in the increased water solubility of 20(S)-Rg3 compared with 20(R)-Rg3 [19,20].
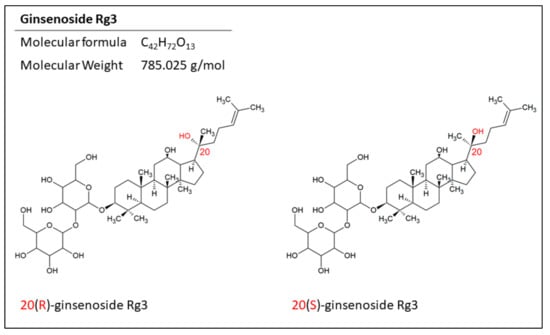
Figure 2.
The structure of the epimers of ginsenoside Rg3. The position of the hydrogen on C20 makes two epimers for this molecule.
3. Epimers of Ginsenoside Rg3 in the Treatment of Cancer
Depending on the biological system being tested, the two epimers of ginsenoside Rg3, 20(S)- and 20(R)-Rg3, have distinct effects. For example, while both epimers inhibited the 5-HT3A and α3β4 nACh receptors, only 20(S)-Rg3 inhibited the voltage-dependent Ca2+, K+, and Na+ channel currents [21,22]. The 20(S)-Rg3 was also a better scavenger of hydroxyl radicals [23] and in the human gastric cancer cell line AGS, was responsible for inducing apoptosis (through activation of caspase-3, -8, and -9) [24]. In human hepatocellular carcinoma cell line HepG2, 20(S)-Rg3 was the more effective epimer in inhibiting cell growth, downregulating the expression of DNA methyltransferases, reducing global DNA methylation, and in particular, modifying the methylation of the promoter region of some relevant genes in cancer such as VEGF, TP53, and BCL-2 [25].
In contrast, 20(R)-Rg3 was a better antioxidant against the oxidative stress induced by cyclophosphamide in mice [26] and can promote the immune response in mice better than 20(S)-Rg3 [27,28]. It was also a better inhibitor of tumor growth in mice bearing H22-transplanted hepatocellular tumors [28]. Epimers of Rg3 have also been tested in epithelial–mesenchymal transition (EMT) in lung adenocarcinoma in vitro models. For instance, 20(R)-Rg3 epimer inhibited EMT via increasing the expression of E-cadherin and inhibiting the expression of vimentin and upregulation of Snail [29].
4. Mechanisms of Action of Ginsenoside Rg3 in Breast Cancer
Regardless of the stereotype, Rg3 has been studied in several cancer models and various mechanisms are suggested for its actions. These mechanisms include induction of apoptosis [24,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52], induction of autophagy through upregulation of autophagy-associated molecules [53], inhibition of proliferation [24,25,38,41,42,44,45,46,50,51,54,55,56,57,58,59,60,61,62,63], inhibition of metastasis [29,50,62,64,65,66,67,68,69,70,71] and angiogenesis [55,56,66], cell cycle arrest [47], immunomodulatory effects [72], sensitization to radiation [73], reducing multidrug resistance [74], and inducing genotoxicity to the cancer cells [75]. A few studies have focused on the effects of Rg3 in breast cancer models; these mechanisms are discussed as follows.
4.1. Induction of Apoptosis and Inhibition of Proliferation
Induction of apoptosis is one of the most studied mechanisms of action of Rg3 in different cancers. Apoptosis is a complex process, regulated by extrinsic (via the death receptor) and intrinsic (via mitochondrial) pathways. The intrinsic pathway is activated by DNA damage and oxidative stress whilst the extrinsic pathway can be triggered by the activation of the members of the TNF receptor superfamily. Both pathways ultimately activate caspase enzymes.
Caspases can interact with an apoptosis inhibitor such as inhibitors of apoptosis proteins (IAP) and the Bcl-2 family. Caspases can also go through auto-activation and cleave other substrates, one of which is PARP, an important DNA repair enzyme. In a TNBC cell line, MDA-MB-231, Rg3 activated caspase-3, and degraded PARP through the generation of reactive oxygen species (ROS) [30]. In addition, Rg3 caused an increased ratio of pro-apoptotic Bax and the anti-apoptotic Bcl-2 [30]. Also, it inhibited the binding of NF-κB to DNA. NF-κB is a transcription factor that is constitutively active in breast cancer cells and drives further cell cycle progression, proliferation and inhibition of apoptosis. Proteins Akt and ERK are two kinases involved in the activation of NF-κB and it is observed that in MDA-MB-231 cell line, Rg3 inhibited the phosphorylation of Akt and ERK and hence prevented the activation of NF-Κb [31]. P53, a tumor suppressor protein, has a negative regulatory effect on Bcl-2 while mutant P53 can prolong the activation of NF-κB and affect the apoptosis of cancer cells. In MDA-MB-231 cells, Rg3 destabilized mutant P53, suppressed the expression of Bcl-2, and induced apoptosis [31]. Figure 3 summarizes these mechanisms.
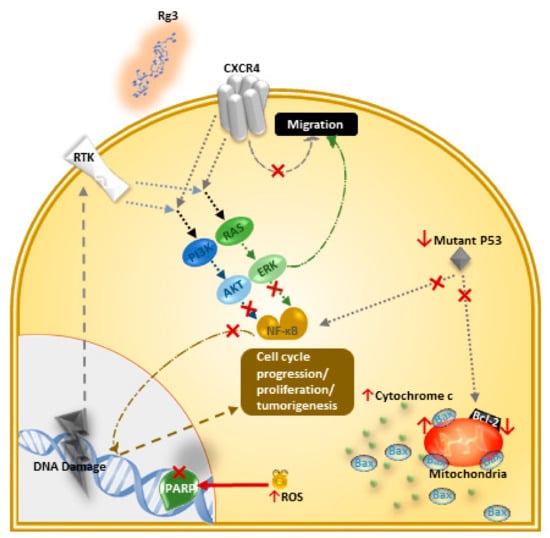
Figure 3.
Rg3 inhibits cell proliferation and induces apoptosis via different effector molecules and pathways, in MDA-MB-231 cell line [30,31]. Changes in specific molecules involved in signalling pathways upon exposure of the cells to Rg3 is shown in this figure. The ↑ and ↓ arrows are indicating increased and decreased levels of certain molecules, respectively, and the × signs show the inhibition of a signalling pathway or function of a certain protein in the MDA-MB-231 cell line.
Inhibition of proliferation is another important function proposed for Rg3. For example, in in vivo settings, Rg3 has shown inhibition of tumor growth in the cancer models of colon [54], lung [38,72], liver [39], pancreas [76], and gallbladder [32]. As a specific epimer, 20(S)-Rg3 has caused similar responses in tumors of the gallbladder [47] and ovary [51,68] and 20(R)-Rg3 in melanoma [61,63], lung [62], and liver [28] cancer models. In vitro, in MCF-7 breast cancer cell lines, 20(S)-Rg3 (100-300 µM), caused a cell cycle arrest in G1-phase and hence inhibited cell proliferation [57]. Table 1 shows other suggested mechanisms of Rg3 in induction of apoptosis and inhibition of proliferation in other cancer models.

Table 1.
Suggested mechanisms for induction of apoptosis (IA) and inhibition of proliferation (IP) by Rg3 in various cancers are summarized in Table 1. The function of different epimers are indicated by symbols; * and ◊ represent 20(S)- and 20(R)-Rg3, respectively.
4.2. Inhibition of Migration, Invasion, Angiogenesis, and Metastasis
Ginsenoside Rg3 has been shown to reduce the migration, invasion, and angiogenesis of human umbilical vein endothelial cells (HUVECs) both in vitro and in vivo. Treatment of HUVECs with 20(R)-Rg3 reduced cell viability with an IC50 of 10 nM. There was a dose-dependent reduction in the tube forming capacity of these cells (1-1000 nM) and inhibition of VEGF-induced chemo-invasion in vitro. In vivo Rg3 inhibited angiogenesis (150 and 600 nM) in a matrigel plug assay. The mechanisms suggested were inhibition of matrix metalloproteinase (MMP)-2 and -9 [77].
Rg3 has been shown to degrade serum levels of IGF-1 and hence inhibits angiogenesis and tumor growth in breast cancer [78]. In a study by Chen et al. [69], in the MDA-MB-231 cell line, 20(S)-Rg3 decreased the expression of CXCR4, an important chemokine receptor expressed by breast cancer cells which is involved in migration and invasion (Figure 3) [69]. Other suggested mechanisms for this action in other cancer models include decreased expression of MMP-2 [29,77], -9 [66], and -13 [67], reducing the expression of HIF-1α [55,68], AQP1 [71], and HDAC3 [63], suppressing NF-κB and its products (c-Myc, COX-2, MMP-9) [55,64], inhibiting TGF-β1, inactivating proteins involved in EMT (p38 MAPK and Smad2) [29,55], downregulating FUT4 and EGFR mediated migration through MAPK and NF-κB [55,62], and decreasing the expression of VEGF [55] and VEGF dependent p38/ERK signaling [56] (Table 2). Rg3 has also resulted in an increased survival in mice bearing melanoma [50] and liver tumors [39].

Table 2.
Suggested mechanisms of inhibition of migration and invasion in different cancer models. The function of different epimers are indicated by symbols; * and ◊ represent 20(S)- and 20(R)-Rg3, respectively.
4.3. Multidrug Resistance (MDR) and Combination Therapy
Rg3 can decrease MDR via inducing membrane fluidity and blocking drug efflux in leukemia cell lines [74]. In the Caco-2 cell line, 20(S)-Rg3 (80 µM) was shown to inhibit P-glycoprotein (Pgp) [79]. It has also been shown to increase the accumulation of drugs such as vincristine in MDR cells, but not in sensitive cells [80]. Likewise, mice bearing MDR tumors showed an increased survival time and less tumor weight when treated with a combination of doxorubicin and Rg3, rather than doxorubicin alone [80].
Few studies have shown the effects of co-administration of Rg3 and a chemotherapy agent in breast tumor models. What is known so far is that mice bearing breast tumors that received a combination of continuous low-dose capecitabine, a prodrug of fluorouracil (5-FU), and Rg3 showed less toxicity induced by capecitabine, longer survival, and reduced susceptibility to drug resistance [81]. This is in part due to the antiangiogenic effect of Rg3 as evidenced by the decreased VEGF expression and reduced microvasculature density. The outcomes of this study are promising for the oral administration of capecitabine and improvement in tolerance of the patients.
In addition, Rg3 when co-administered orally with paclitaxel, significantly increased the relative bioavailability of paclitaxel and decreased the relative breast tumor growth rate in mice bearing MCF-7 xenograft [79]. Rg3 has been tested in other cancer models in combination with cyclophosphamide [82,83], gemcitabine [84], temozolomide [85], cisplatin [86,87,88], docetaxel [89,90], doxorubicin [91,92], and As2O3 [93] (Table 3).

Table 3.
Suggested effects of Rg3 in combination with chemotherapy agents in in vitro and in vivo models.
4.4. Aquaporin (AQP) 1—a Putative Target of Rg3
One suggested mechanism of action of Rg3 is by targeting AQP1 [71]. This molecule has roles in tumor growth, angiogenesis [94,95,96], metastasis [97,98], acquired resistance in tumors [99], and is highly expressed in aggressive tumors [100]. AQP1 is a member of the family of AQP membrane channels which are primarily known for their role in water transport across the lipophilic cell membrane. AQP1 was the first of the 13 members of the AQP proteins to be discovered [95,101,102]. It is a unique AQP in that, as well as acting as a water channel, it has a second function of transporting single charged cations, regulated by cGMP gating. AQP1 also transports gases such as nitric oxide, carbon dioxide, and ammonia (Figure 4) [102,103,104,105]. Rg3 was found to inhibit expression of AQP1 at both the mRNA and protein levels. Further, in a prostate cancer cell line (PC-3M), Rg3 (up to 10 µM) did not affect cell proliferation but inhibited cell migration in a transwell assay. Overexpression of AQP1 in this cell line attenuated the effect of Rg3 in inhibiting migration while silencing AQP1 gene via shRNA resulted in reduced PC-3M cell migration, and a diminished response to Rg3. These results indicated a critical role for AQP1 mediating the anti-migratory role of Rg3 [71]. This suggests that Rg3 targeting AQP1 could also be relevant in breast tumors.
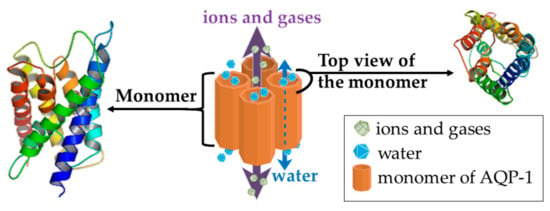
Figure 4.
The structure of AQP1 channel, as a homotetramer, with the dashed arrow showing the water passage through the water channel of each monomer. The solid violet arrow represents the passage of ions and gases. The 3D structures were prepared in PyMol, version 1.7.4.5 (Schrödinger, Inc, Tokyo, Japan).
AQP1 and Breast Cancer
In vitro data suggest that stable overexpression of AQP1 in MCF-7 (ER+ and PR+) and MDA-MB-231 (TNBC) breast cancer cell lines significantly increases cell invasion and proliferation [106]. In HUVECs, expression of AQP1 is known to be upregulated by estrogen, because the promoter of the AQP1 gene has a functional estrogen response element (ERE) and the homodimerized complex of estrogen-ER can activate this ERE [107]. AQP1 is expressed in all microvasculature endothelial cells including HUVECs. In HUVECs, estrogen increased the proliferation, migration, invasion, and tube forming capacity, and these effects were inhibited by knockdown of AQP1 expression using siRNA [107]. Epidermal growth factor stimulation induced translocation of AQP1 from the cytoplasm to the cell membrane to enhance cell invasion [106]. AQP1 was also found to colocalize with ezrin, a cytoskeletal protein involved in the proliferation, cell adhesion, and NO production in the endothelial cells [107].
Animal studies show that AQP1 is highly expressed in mouse breast tumor [108], and AQP1-null mice show impaired angiogenesis [109]. In mouse models of breast carcinoma with lung metastasis, AQP1 deficiency decreased the expression of VEGFR2 leading to significantly reduced tumor mass and volume, microvasculature density, and the number of lung metastases [110].
In humans, AQP1 is abundantly expressed in the endothelium of many tissues [111] including the endothelium of tumor micro-vasculature, positive for CD31 [100,107]. In normal breast tissues, the expression of AQP1 is low and limited to the ducts, lymphatics and connective tissue microvessels [112]. Breast cancer is one of the tumors with increased microvessels and angiogenesis compared to its matched normal tissue and there is an increased AQP1 expression in the microvasculature of breast tumor [112,113]. Breast tumors of basal-like TNBC subtype and advanced breast tumors have higher levels of AQP1 expression compared to normal tissues [112,113,114]. Benign breast lesions and ductal carcinoma in situ samples express AQP1 on the membrane of myoepithelial cells of the ducts, but the majority of invasive ductal carcinoma samples predominantly express AQP1 in the cytoplasm [106,115]. So far, studies have shown that membrane AQP1 expression is associated with triple-negativity, expression of cytokeratin 14 and smooth muscle actin, higher tumor grade, medullary-like histology and poor clinical prognosis [113,114]. High AQP1 expression was found to be an independent prognostic factor in the high-grade subgroup, in the ER-negative subgroup and in the node-negative subgroup [114]. Cytoplasmic expression of APQ1 is correlated with lymph node metastasis and advanced features of invasive ductal carcinoma [106].
4.5. Other Suggested Mechanisms of Action
Rg3, when orally administered to mice bearing lung tumors, had immunomodulatory effects, causing increased splenocyte proliferation [72]. It also increased genotoxicity in osteosarcoma cell lines, through increased DNA damage and double-strand breaks [75]. This compound sensitized lung cancer tumors to radiation via suppressing the activation of NF-κB and the proteins regulated by this transcription factor (such as COX2, MMP-9, VEGF, c-Myc, and cyclin D1), which are either induced by radiation or are involved in radio-resistance [73].
5. Metabolism and Pharmacokinetics of Rg3
Together with the clinical trials, it is pertinent to consider the pharmacokinetics of Rg3 following oral administration. So far, various studies have focused on the metabolism and pharmacokinetics of Rg3, as a general compound, and 20(R)-Rg3 in in vitro, animal models and healthy human volunteers. It is not yet clarified whether the metabolism of 20(S)- and 20(R)-Rg3 differ in any aspects. The general understanding is that following oral administration, ginsenosides undergo a partial or complete hydrolysis in the acidic conditions of the stomach and the intestinal microbial flora [116,117]. Rg3, like other protopanaxadiol ginsenosides, can lose a sugar moiety following metabolism by the anaerobic intestinal bacteria [118]. Compound K, the final metabolite of the metabolism of ginsenosides can be detected in human plasma after seven hours post-ingestion [118].
In vitro studies have shown that Rg3 has interactions with isoenzymes of cytochrome P450. Rg3 can weakly inhibit CYP3A4, moderately inhibit CYP2C19 and CYP1A2, and potently inhibit CYP2D4 [119] and so interactions between Rg3 and the drugs that are mainly metabolized with these isoenzymes should be considered. Also, incubation of Rg3 with human fecal microflora resulted in the formation of ginsenoside Rh2 [120], another member of the ginsenoside family with anticancer properties [121]. Studies in dogs however failed to detect any Rh2 in the plasma samples following oral or intravenous (IV) administration of 20(R)-Rg3 [122]. Although in vitro studies suggest that deglycosylation is one of the main pathways of the metabolism of Rg3, this study failed to show existence of such molecules in dog plasma samples [122]. This study also suggested a low degree of metabolism of 20(R)-Rg3, as evidenced by the maximum of 70% of 20(R)-Rg3 recovered from bile [122].
In rats, deglycosylation and oxygenation are reported as two major routes of metabolism for 20(R)-Rg3 [123]. The half-life of Rg3 in rats after an intravenous administration is reported to be 14 min [124] and 18.5 min [123]. The difference between these two reports might be due to the difference in the solubilisation of Rg3, however, both suggest a rapid rate of metabolic clearance for this molecule. Absolute bioavailablility of Rg3 in rats was about 2.63% [125].
Intra-species differences seem to play an important role in the metabolism and pharmacokinetics of Rg3, since in healthy human volunteers, Rg3 can be detected in the plasma for 8 [126,127] and up to 216 hours [126], following oral and intramuscular (IM) administration, respectively.
The epimers of Rg3 also differ in terms of tissue distribution. 20(S)-Rg3, following oral administration of 68 mg/kg to Sprague–Dawley rats was more concentrated in the gastrointestinal tissues compared to the plasma. It was also highly distributed in the liver, with the concentration being four and three times the plasma concentration at two and four hours, respectively. The concentration of Rg3 in other tissues such as muscle, spleen, lung, and fat was similar or lower than plasma concentration and trace amounts were detected in the brain, heart, and kidney. However, 20(R)-Rg3 was only localized in liver and the gastrointestinal tract, and not detected in the plasma [128]. Table 4 summarizes the results of the studies on the pharmacokinetics of Rg3 in animal models and human trials.

Table 4.
A summary of the studies on the pharmacokinetics of Rg3 and the 20(R) epimer in in vivo models.
6. Clinical Trials
6.1. Application and Safety of Ginseng Extract on Healthy Human Volunteers
Studies on healthy human volunteers suggest that administration of the total extract of Panax ginseng C.A. Meyer is well tolerated and does not cause serious adverse reactions [130,131]. In a randomized, double-blind, placebo-controlled trial investigating the anti-oxidant properties of the total extract, 82 healthy volunteers received either placebo (n = 27), 1 or 2 g/day (n = 27 and n = 28, respectively) for a month [130]. Of the 82 volunteers, 80 completed the trial; only two, both female, randomized to receive 2 g/day of the total extract withdrew, one due to insomnia and palpitations after seven days, and the other due to non-health related reasons. Administration of the total extract improved the serum levels of anti-oxidant markers. In another randomized, double-blind, placebo-controlled trial investigating the anti-oxidant effects in postmenopausal women, 41 volunteers received placebo and 41 received 1 g of the extract thrice daily for 12 weeks [131]. Five volunteers receiving placebo and six receiving the extract failed to complete the trial. Administration of the total extract increased the enzyme activity of the serum antioxidant, superoxide dismutase, suggesting that the total extract may reduce oxidative stress in postmenopausal women. At this dose, reported side effects included dizziness, sleeplessness, nervousness, and uterine bleeding [131]. Adverse effects following administration of 1 or 2 g/day of total extract of ginseng to healthy subjects for a month were reported to be mild (constipation and dyspepsia, insomnia, and hot flash) and it was concluded that this extract does not cause serious adverse reactions and is safe and tolerable [132].
6.2. Clinial Trials and Application of Rg3 in Cancer Patients
Presently, there are only three published clinical trials utilizing Rg3 in the treatment of cancer; two on non-small cell lung carcinoma (NSCLC) [95] [96] and one on hepatocellular carcinoma (HCC) [99]. In the first study, a total of 133 patients with stage II-III NSCLC received either Rg3 alone (43 cases), Rg3 + chemotherapy (46 cases), or chemotherapy alone (44 cases) [133]. Rg3 was administered twice a day (0.8 mg/kg, equivalent to 40–50 mg/day) for at least 6 months. This study showed that Rg3 + chemotherapy improved the 3-year survival rates compared to either Rg3 or chemotherapy alone (54.3% versus either 46.5% or 47.7%, respectively; p > 0.05). In patients expressing VEGF, chemotherapy treatment alone resulted in decreased 3-year survival rates compared to patients with negative VEGF expression (p < 0.01); however, there were no significant differences for the other two groups. In addition, patients that received Rg3 had a lower incidence of adverse effects and better immune system function, as evidenced by the increased activity of NK cells and CD4+ T cells and the normal ratio of CD4+/CD8+ T cells [133]. This suggests that the option of combining Rg3 therapy with immunotherapy would be worth investigating.
In the second study, 124 patients with advanced (stage III-IV), unresectable NSCLC with EGFR mutations were divided into two groups receiving a tyrosine kinase inhibitor (TKI) + Rg3 (20 mg orally for at least 2 months) or TKI alone [134]. The results of this study demonstrated that Rg3 improved the median progression-free survival by 2.5 months (p = 0.049). Rg3 delayed the acquired resistance to TKI and had a low toxicity profile, with rash being the worst side effect in both groups and nausea, diarrhea, and anorexia being the most common side effects in both groups [134].
In the third study, 228 patients diagnosed with advanced (Barcelona clinic liver cancer-stage C) HCC were randomized in two groups, to receive trans-arterial chemoembolization (TACE) alone or in combination with Rg3 (20 mg, twice a day, orally) [135]. TACE is a successful method for delivering chemotherapy directly to the tumor within the liver which prolongs patient survival, but its application is limited by high recurrence rate, in part due to inflammatory factors promoting metastasis of the tumor. Inflammation and angiogenesis are associated phenomena in that pro-inflammatory cytokines such as IL-1ß or TNF-α released from activated neutrophils and macrophages cause vasculature modifications, enhancing proliferation of endothelial cells and hyper-neovascularization [136,137]. Hence, using an anti-angiogenic drug should limit this adverse effect. This study showed that the patients receiving TACE + Rg3 had longer median overall survival compared to those who received TACE alone (13.2 versus 10 months; p = 0.002), while there was no significant difference in progression free survival. Rg3 was well-tolerated, the reported adverse effects being grade 1 or 2 constipation, epistaxis, and hypertension, and importantly, Rg3 treatment tended to alleviate adverse effects related to TACE [135].
7. Conclusions
So far, many studies have shown the effects of Rg3 in different cancer models with fewer studies in human clinical trials. A limited number of studies have focused on the effects of Rg3 in breast cancer models and more specifically in advanced breast cancer. Out of the six studies on the effects of Rg3 in breast cancer, only one study has focused on the effects of an isomer, 20(S)-Rg3, and its in vivo effects in mice in increasing the efficacy of oral paclitaxel [79]. The rest of these studies used Rg3 as a whole compound [30,31,69,81,138]. In some cases, the source of the Rg3 used is self-produced, and of unknown purity [30,31]. Given the fact that Rg3 can have stereospecific activities [24,28,29], a mixture of two enantiomers, with unknown ratios of each enantiomer, cannot scientifically justify the resulting effects. With this view, stereospecific activity of Rg3 in human breast cancer models, including cell lines and patient tumor-derived cancer cells, in 2D and 3D in vitro models, and in vivo, is not known. Furthermore, considering the importance of AQP1 in angiogenesis and the invasiveness of tumors, together with evidence of survival benefit from clinical trials, the selectivity of Rg3 in targeting AQP1 in metastatic breast tumors should be studied.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, N. Platinum resistance in breast and ovarian cancer cell lines. J. Exp. Clin. Cancer Res. 2011, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Harlan, L.C.; Dodd, K.W.; Abrams, J.S.; Ballard-Barbash, R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Investig. 2010, 28, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Fischer, O.M.; Streit, S.; Hart, S.; Ullrich, A. Beyond herceptin and gleevec. Curr. Opin. Chem. Biol. 2003, 7, 490–495. [Google Scholar] [CrossRef]
- Elias, A.D. Triple-negative breast cancer: A short review. Am. J. Clin. Oncol. 2010, 33, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, S. Systemic Therapy. In Breast Cancer; Mahon, S.M., Ed.; Oncology Nursing Society: Pittsburgh, PA, USA, 2011. [Google Scholar]
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Robert, N.; Leyland-Jones, B.; Asmar, L.; Belt, R.; Ilegbodu, D.; Loesch, D.; Raju, R.; Valentine, E.; Sayre, R.; Cobleigh, M. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2–overexpressing metastatic breast cancer. J. Clin. Oncol. 2006, 24, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.-S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Park, J.D.; Rhee, D.K.; Lee, Y.H. Biological activities and chemistry of saponins from Panax ginseng CA Meyer. Phytochem. Rev. 2005, 4, 159–175. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, D.H.; Park, S.J.; Kim, J.M.; Ryu, J.H. Ginseng in traditional herbal prescriptions. J. Ginseng Res. 2012, 36, 225. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.S.; Wu, M.Y. Chinese ginseng. In Nutraceuticals; Elsevier: New York City, NY, USA, 2016; pp. 693–705. [Google Scholar]
- Chang-Xiao, L.; Pei-Gen, X. Recent advances on ginseng research in China. J. Ethnopharmacol. 1992, 36, 27–38. [Google Scholar] [CrossRef]
- Choi, K.-T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol. Sin. 2008, 29, 1109. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Aung, H.H.; Ni, M.; Wu, J.-A.; Tong, R.; Wicks, S.; He, T.-C.; Yuan, C.-S. Red American ginseng: Ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007, 73, 669. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, C.-Z.; Tong, R.; Li, X.-L.; Fishbein, A.; Wang, Q.; He, T.-C.; Du, W.; Yuan, C.-S. Effects of steaming the root of Panax notoginseng on chemical composition and anticancer activities. Food Chem. 2010, 118, 307–314. [Google Scholar] [CrossRef]
- Chang, Y.H.; Ng, P.K. Effects of Extrusion process variables on extractable ginsenosides in wheat-ginseng extrudates. J. Agric. Food Chem. 2009, 57, 2356–2362. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Chen, F. Degradation of ginsenosides in American ginseng (Panax quinquefolium) extracts during microwave and conventional heating. J. Agric. Food Chem. 1999, 47, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-I.; Lee, J.-Y.; Yang, J.-Y.; Jeong, S.M.; Lee, J.-H.; Nah, S.-Y.; Kim, Y. Evidence that the tertiary structure of 20 (S)-ginsenoside Rg 3 with tight hydrophobic packing near the chiral center is important for Na+ channel regulation. Biochem. Biophys. Res. Commun. 2005, 333, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-W.; Wang, C.-Z.; Yuan, C.-S. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Lee, J.-H.; Kim, J.-H.; Lee, B.-H.; Yoon, I.-S.; Lee, J.-H.; Kim, D.-H.; Rhim, H.; Kim, Y.; Nah, S.-Y. Stereospecificity of Ginsenoside Rg 3 Action on Ion Channels. Mol Cells 2004, 18, 383–389. [Google Scholar] [PubMed]
- Kim, J.-H.; Lee, J.-H.; Jeong, S.M.; Lee, B.-H.; Yoon, I.-S.; Lee, J.-H.; Choi, S.-H.; Kim, D.-H.; Park, T.-K.; Kim, B.-K. Stereospecific effects of ginsenoside Rg3 epimers on swine coronary artery contractions. Biol. Pharm. Bull. 2006, 29, 365–370. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.Y.; Kang, K.S.; Lee, J.G.; Yokozawa, T.; Park, J.H. The chemical and hydroxyl radical scavenging activity changes of ginsenoside-Rb1 by heat processing. Bioorg. Med. Chem. Lett. 2008, 18, 4515–4520. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-H.; Kim, Y.-J.; Yamabe, N.; Park, S.-H.; Kim, H.-K.; Jang, H.-J.; Kim, J.H.; Cheon, G.J.; Ham, J.; Kang, K.S. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J. Ginseng Res. 2014, 38, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Wang, Y.; Li, P.; Liu, J.; Wei, A.; Wang, H.; Meng, X.; Pan, D.; Zhang, X. Effects of R type and S type ginsenoside Rg3 on DNA methylation in human hepatocarcinoma cells. Mol. Med. Rep. 2017, 15, 2029–2038. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Su, F.; Su, X.; Hu, T.; Hu, S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia 2012, 83, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, J.; Su, F.; Su, X.; Hu, T.; Hu, S. Stereospecificity of ginsenoside Rg3 in promotion of the immune response to ovalbumin in mice. Int. Immunol. 2012, 24, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ru, Q.; Chen, L.; Ma, B.; Li, C. Stereospecificity of Ginsenoside Rg3 in the Promotion of Cellular Immunity in Hepatoma H22-Bearing Mice. J. Food Sci. 2014, 79, H1430–H1435. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Choi, W.-I.; Jeon, B.-N.; Choi, K.-C.; Kim, K.; Kim, T.-J.; Ham, J.; Jang, H.J.; Kang, K.S.; Ko, H. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial–mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology 2014, 322, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-M.; Kim, D.-H.; Park, J.-H.; Na, H.-K.; Surh, Y.-J. Ginsenoside Rg3 induces apoptosis of human breast cancer (MDA-MB-231) cells. J. Cancer Prev. 2013, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-M.; Kim, D.-H.; Park, J.-H.; Surh, Y.-J.; Na, H.-K. Ginsenoside Rg3 inhibits constitutive activation of NF-κB signaling in human breast cancer (MDA-MB-231) cells: ERK and Akt as potential upstream targets. J. Cancer Prev. 2014, 19, 23. [Google Scholar] [CrossRef]
- Wu, K.; Li, N.; Sun, H.; Xu, T.; Jin, F.; Nie, J. Endoplasmic reticulum stress activation mediates Ginseng Rg3-induced anti-gallbladder cancer cell activity. Biochem. Biophys. Res. Commun. 2015, 466, 369–375. [Google Scholar] [CrossRef]
- Aziz, F.; Wang, X.; Liu, J.; Yan, Q. Ginsenoside Rg3 induces FUT4-mediated apoptosis in H. pylori CagA-treated gastric cancer cells by regulating SP1 and HSF1 expressions. Toxicol. In Vitro 2016, 31, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Nah, S.Y.; Jeon, J.H.; So, I.; Kim, S.J. Transient Receptor Potential Melastatin 7 Channels are Involved in Ginsenoside Rg3-Induced Apoptosis in Gastric Cancer Cells. Basic Clin. Pharmacol. Toxicol. 2011, 109, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, P.; Zeng, H.Q.; Lou, S.F.; Wang, D.X. Ginsenoside Rg3 induces apoptosis in human multiple myeloma cells via the activation of Bcl-2-associated X protein. Mol. Med. Rep. 2015, 12, 3557–3562. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Tian, L.L.; Zhang, Y.M.; Wang, X.Q.; Yan, Q.; Liu, J.W. Ginsenoside Rg3 suppresses FUT4 expression through inhibiting NF-κB/p65 signaling pathway to promote melanoma cell death. Int. J. Oncol. 2015, 47, 701–709. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, H.J.; Kang, D.W.; Han, I.H.; Choi, B.K.; Cho, W.H. Ginsenoside Rg3 induces apoptosis in the U87MG human glioblastoma cell line through the MEK signaling pathway and reactive oxygen species. Oncol. Rep. 2013, 30, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.J.; Chun, J.; Ha, Y.W.; Ko, H.J.; Xu, M.-Y.; Kim, Y.S. Novel roles of ginsenoside Rg3 in apoptosis through downregulation of epidermal growth factor receptor. Chem. Biol. Interact. 2015, 233, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-W.; Chen, X.-M.; Chen, X.-H.; Zheng, S.-S. Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via intrinsic apoptotic pathway. World J. Gastroenterol. 2011, 17, 3605. [Google Scholar] [CrossRef]
- Xie, Q.; Wen, H.; Zhang, Q.; Zhou, W.; Lin, X.; Xie, D.; Liu, Y. Inhibiting PI3K-AKt signaling pathway is involved in antitumor effects of ginsenoside Rg3 in lung cancer cell. Biomed. Pharmacother. 2017, 85, 16–21. [Google Scholar] [CrossRef]
- Sun, H.Y.; Lee, J.H.; Han, Y.-S.; Yoon, Y.M.; Yun, C.W.; Kim, J.H.; Song, Y.S.; Lee, S.H. Pivotal roles of ginsenoside Rg3 in tumor apoptosis through regulation of reactive oxygen species. Anticancer Res. 2016, 36, 4647–4654. [Google Scholar] [CrossRef]
- Li, Y.; Yang, T.; Li, J.; Hao, H.L.; Wang, S.Y.; Yang, J.; Luo, J.M. Inhibition of multiple myeloma cell proliferation by ginsenoside Rg3 via reduction in the secretion of IGF-1. Mol. Med. Rep. 2016, 14, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Jung, K.H.; Morgan, M.J.; Kang, Y.-R.; Lee, H.-S.; Koo, G.-B.; Hong, S.-S.; Kwon, S.W.; Kim, Y.-S. Sensitization of TRAIL-induced cell death by 20 (S)-ginsenoside Rg3 via CHOP-mediated DR5 upregulation in human hepatocellular carcinoma cells. Mol. Cancer Ther. 2013, 12, 274–285. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Yu, Y.; Chen, B.; Tang, C.; Li, X. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Mol. Med. Rep. 2012, 5, 1295–1298. [Google Scholar] [PubMed]
- Yuan, H.-D.; Quan, H.-Y.; ZHanG, Y.; KiM, S.H.; Chung, S.-H. 20 (S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Mol. Med. Rep. 2010, 3, 825–831. [Google Scholar] [PubMed]
- Lee, S.Y.; Kim, G.T.; Roh, S.H.; Song, J.-S.; Kim, H.-J.; Hong, S.-S.; Kwon, S.W.; Park, J.H. Proteomic analysis of the anti-cancer effect of 20S-ginsenoside Rg3 in human colon cancer cell lines. Biosci. Biotechnol. Biochem. 2009, 73, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, M.; Wu, X.; Hu, Y.; Cao, Y.; Wang, X.A.; Xiang, S.; Li, H.; Jiang, L.; Tan, Z. 20 (S)-ginsenoside Rg3 promotes senescence and apoptosis in gallbladder cancer cells via the p53 pathway. Drug Des. Devel. Ther. 2015, 9, 3969. [Google Scholar] [PubMed]
- Qiu, X.-M.; Bai, X.; Jiang, H.-F.; He, P.; Wang, J.-H. 20-(s)-ginsenoside Rg3 induces apoptotic cell death in human leukemic U937 and HL-60 cells through PI3K/Akt pathways. Anticancer Drugs 2014, 25, 1072–1080. [Google Scholar] [CrossRef]
- Park, H.-M.; Kim, S.-J.; Kim, J.-S.; Kang, H.-S. Reactive oxygen species mediated ginsenoside Rg3-and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food Chem. Toxicol. 2012, 50, 2736–2741. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Ou-Yang, X.; He, X. Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma cells. Melanoma Res. 2008, 18, 322–329. [Google Scholar] [CrossRef]
- Li, J.; Liu, T.; Zhao, L.; Chen, W.; Hou, H.; Ye, Z.; Li, X. Ginsenoside 20 (S)-Rg3 inhibits the Warburg effect through STAT3 pathways in ovarian cancer cells. Int. J. Oncol. 2015, 46, 775–781. [Google Scholar] [CrossRef]
- Wang, J.-H.; Nao, J.-F.; Zhang, M.; He, P. 20 (s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol. 2014, 35, 11985–11994. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, W.; Hou, H.; Li, J.; Li, H.; Sun, X.; Zhao, L.; Li, X. Ginsenoside 20 (S)-Rg3 induced autophagy to inhibit migration and invasion of ovarian cancer. Biomed. Pharmacother. 2017, 85, 620–626. [Google Scholar] [CrossRef] [PubMed]
- He, B.-C.; Gao, J.-L.; Luo, X.; Luo, J.; Shen, J.; Wang, L.; Zhou, Q.; Wang, Y.-T.; Luu, H.H.; Haydon, R.C. Ginsenoside Rg3 inhibits colorectal tumor growth through the down-regulation of Wnt/ss-catenin signaling. Int. J. Oncol. 2011, 38, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-J.; Zhang, M.-Z.; Wang, L.-X. Gensenoside Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells. Cell. Physiol. Biochem. 2010, 26, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Jung, S.-Y.; Kwon, Y.-H.; Lee, J.-H.; Lee, Y.M.; Lee, B.-Y.; Kwon, S.-M. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol. Ther. 2012, 13, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Aung, H.H.; Zhang, B.; Sun, S.; Li, X.-L.; He, H.; Xie, J.-T.; He, T.-C.; Du, W.; Yuan, C.-S. Chemopreventive effects of heat-processed Panax quinquefolius root on human breast cancer cells. Anticancer Res. 2008, 28, 2545–2551. [Google Scholar] [PubMed]
- Luo, X.; Wang, C.-Z.; Chen, J.; Song, W.-X.; Luo, J.; Tang, N.; He, B.-C.; Kang, Q.; Wang, Y.; Du, W. Characterization of gene expression regulated by American ginseng and ginsenoside Rg3 in human colorectal cancer cells. Int. J. Oncol. 2008, 32, 975–983. [Google Scholar] [CrossRef]
- Sin, S.; Kim, S.Y.; Kim, S.S. Chronic treatment with ginsenoside Rg3 induces Akt-dependent senescence in human glioma cells. Int. J. Oncol. 2012, 41, 1669–1674. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, E.-H.; Ko, S.-R.; Choi, K.-J.; Park, J.-H.; Im, D.-S. Effects of ginsenosides Rg 3 and Rh 2 on the proliferation of prostate cancer cells. Arch. Pharm. Res. 2004, 27, 429. [Google Scholar] [CrossRef]
- Shan, X.; Aziz, F.; Tian, L.L.; Wang, X.Q.; Yan, Q.; Liu, J.W. Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation inhibits melanoma cell proliferation by decreasing FUT4/LeY expression. Int. J. Oncol. 2015, 46, 1667–1676. [Google Scholar] [CrossRef]
- Tian, L.; Shen, D.; Li, X.; Shan, X.; Wang, X.; Yan, Q.; Liu, J. Ginsenoside Rg3 inhibits epithelial-mesenchymal transition (EMT) and invasion of lung cancer by down-regulating FUT4. Oncotarget 2016, 7, 1619. [Google Scholar] [CrossRef]
- Shan, X.; Fu, Y.-S.; Aziz, F.; Wang, X.-Q.; Yan, Q.; Liu, J.-W. Ginsenoside Rg3 inhibits melanoma cell proliferation through down-regulation of histone deacetylase 3 (HDAC3) and increase of p53 acetylation. PLoS ONE 2014, 9, e115401. [Google Scholar] [CrossRef] [PubMed]
- Junmin, S.; Hongxiang, L.; Zhen, L.; Chao, Y.; Chaojie, W. Ginsenoside Rg3 inhibits colon cancer cell migration by suppressing nuclear factor kappa B activity. J. Tradit. Chin. Med. 2015, 35, 440–444. [Google Scholar] [CrossRef]
- Shinkai, K.; Akedo, H.; Mukai, M.; Imamura, F.; Isoai, A.; Kobayashi, M.; Kitagawa, I. Inhibition of in vitro tumor cell invasion by ginsenoside Rg3. Cancer Sci. 1996, 87, 357–362. [Google Scholar]
- Tm, X.; Man-hua, C.; Xin, Y.; Lp, G. Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin. Med. J. 2008, 121, 1394–1397. [Google Scholar]
- Lee, S.G.; Kang, Y.J.; Nam, J.-O. Anti-metastasis effects of ginsenoside Rg3 in B16F10 cells. J. Microbiol. Biotechnol. 2015, 25, 1997–2006. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, L.; Zhang, Y.; Chen, W.; Liu, D.; Hou, H.; Ding, L.; Li, X. Ginsenoside 20 (S)-Rg3 targets HIF-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS ONE 2014, 9, e103887. [Google Scholar] [CrossRef]
- Chen, X.-P.; Qian, L.-L.; Jiang, H.; Chen, J.-H. Ginsenoside Rg3 inhibits CXCR 4 expression and related migrations in a breast cancer cell line. Int. J. Clin. Oncol. 2011, 16, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yoo, Y.; Matsuzawa, K.; Sato, K.; Saiki, I.; Tonooka, S.; Samukawa, K.; Azuma, I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20 (R)-and 20 (S)-ginsenoside-Rg3, of red ginseng. Biol. Pharm. Bull. 1995, 18, 1197–1202. [Google Scholar] [CrossRef]
- Pan, X.-Y.; Guo, H.; Han, J.; Hao, F.; An, Y.; Xu, Y.; Xiaokaiti, Y.; Pan, Y.; Li, X.-J. Ginsenoside Rg3 attenuates cell migration via inhibition of aquaporin 1 expression in PC-3M prostate cancer cells. Eur. J. Pharmacol. 2012, 683, 27–34. [Google Scholar] [CrossRef]
- Park, D.; Bae, D.-K.; Jeon, J.H.; Lee, J.; Oh, N.; Yang, G.; Yang, Y.-H.; Kim, T.K.; Song, J.; Lee, S.H. Immunopotentiation and antitumor effects of a ginsenoside Rg3-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ. Toxicol. Pharmacol. 2011, 31, 397–405. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Song, Y.M.; Wang, B.; Zhang, F.R.; Yang, R.; Wang, H.Q.; Zhang, G.J. Ginsenoside Rg3 sensitizes human non-small cell lung cancer cells to γ-radiation by targeting the nuclear factor-κB pathway. Mol. Med. Rep. 2015, 12, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-Y.; Kim, E.-H.; Kim, S.-W.; Kim, S.-N.; Park, J.-D.; Rhee, D.-K. Selective toxicity of ginsenoside Rg 3 on multidrug resistant cells by membrane fluidity modulation. Arch. Pharm. Res. 2008, 31, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Li, H.-D.; Li, B.; Jiang, S.-D.; Jiang, L.-S. Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells and reduces MNNG-induced DNA damage and apoptosis in normal human cells. Oncol. Rep. 2014, 31, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-Q.; Zheng, Q.-H.; Chen, H.; Chen, L.; Xu, J.-B.; Chen, M.-Y.; Lu, D.; Wang, Z.-H.; Tong, H.-F.; Lin, S. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int. J. Oncol. 2014, 45, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.Y.; Wong, D.Y.; Wu, P.; Leung, P.; Mak, N.; Yeung, H.; Liu, L.; Cai, Z.; Jiang, Z.-H.; Fan, T. The angiosuppressive effects of 20 (R)-ginsenoside Rg3. Biochem. Pharmacol. 2006, 72, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ren, Y.; Zhang, J.; Ma, S.; Gao, F.; Wu, Y. Correlation of insulin-like growth factor-1 (IGF-1) to angiogenesis of breast cancer in IGF-1-deficient mice. Ai Zheng 2007, 26, 1215–1220. [Google Scholar] [PubMed]
- Yang, L.Q.; Wang, B.; Gan, H.; Fu, S.T.; Zhu, X.X.; Wu, Z.N.; Zhan, D.W.; Gu, R.L.; Dou, G.F.; Meng, Z.Y. Enhanced oral bioavailability and anti-tumour effect of paclitaxel by 20 (s)-ginsenoside Rg3 in vivo. Biopharm. Drug Dispos. 2012, 33, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Kwon, H.-Y.; Chi, D.-W.; Shim, J.-H.; Park, J.-D.; Lee, Y.-H.; Pyo, S.; Rhee, D.-K. Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg3. Biochem. Pharmacol. 2003, 65, 75–82. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, X.; Yang, B.; Wang, J.; Yang, F. Antiangiogenic effect of capecitabine combined with ginsenoside Rg3 on breast cancer in mice. Cancer Biother. Radiopharm. 2008, 23, 647–654. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, X.; Zhao, W. Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem. Biophys. Res. Commun. 2006, 342, 824–828. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Wu, C.F.; Duan, L.; Yang, J.Y. Protective effects of ginsenoside Rg 3 against cyclophosphamide-induced DNA damage and cell apoptosis in mice. Arch. Toxicol. 2008, 82, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-G.; Huang, Y.; Cui, D.-D.; Huang, X.-B.; Mao, S.-H.; Ji, L.-L.; Song, H.-B.; Yi, C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer 2009, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yu, Y.; Wang, L.; Wu, B.; Xia, L.; Feng, F.; Ling, Z.; Wang, S. Additive antiangiogenesis effect of ginsenoside Rg3 with low-dose metronomic temozolomide on rat glioma cells both in vivo and in vitro. J. Exp. Clin. Cancer Res. 2016, 35, 32. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Park, K.-K.; Chung, A.-S.; Chung, W.-Y. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/NAD (P) H quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food Chem. Toxicol. 2012, 50, 2565–2574. [Google Scholar] [PubMed]
- Lee, Y.J.; Lee, S.; Ho, J.N.; Byun, S.-S.; Hong, S.K.; Lee, S.E.; Lee, E. Synergistic antitumor effect of ginsenoside Rg3 and cisplatin in cisplatin-resistant bladder tumor cell line. Oncol. Rep. 2014, 32, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Huo, B.; Lv, Y.; Wang, Y.; Liu, W. Ginsenoside Rg3 enhances the inhibitory effects of chemotherapy on esophageal squamous cell carcinoma in mice. Mol. Clin. Oncol. 2014, 2, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, S.Y.; Yuk, D.Y.; Moon, D.C.; Choi, S.S.; Kim, Y.; Han, S.B.; Oh, K.-W.; Hong, J.T. Inhibition of NF-κB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch. Pharm. Res. 2009, 32, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Lee, S.Y.; Cho, J.S.; Son, S.M.; Choi, S.S.; Yun, Y.P.; Yoo, H.S.; Oh, K.-W.; Han, S.B.; Hong, J.T. Combination of ginsenoside Rg3 with docetaxel enhances the susceptibility of prostate cancer cells via inhibition of NF-κB. Eur. J. Pharmacol. 2010, 631, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Jung, K.H.; Lee, D.-G.; Yoon, J.-H.; Choi, K.S.; Kwon, S.W.; Shen, H.-M.; Morgan, M.J.; Hong, S.-S.; Kim, Y.-S. 20 (S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget 2014, 5, 4438. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Wang, T.; Jiang, X.; Zhang, H.; Li, P.; Lv, B.; Gao, X. Ginsenoside Rg3 antagonizes adriamycin-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine 2015, 22, 875–884. [Google Scholar] [CrossRef]
- Che, J.-B.; Liu, Z.-H.; Ma, H.-B.; Li, Y.; Zhao, H.; Li, X.-H.; Liu, W.-C.; Shi, G.-N. Influence of As2O3 combined with ginsenosides Rg3 on inhibition of lung cancer NCI-H1299 cells and on subsistence of nude mice bearing hepatoma. Asian Pac. J. Trop. Med. 2014, 7, 772–775. [Google Scholar] [CrossRef]
- Yool, A.J.; Brown, E.A.; Flynn, G.A. Roles for novel pharmacological blockers of aquaporins in the treatment of brain oedema and cancer. Clin. Exp. Pharmacol. Physiol. 2010, 37, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J. Functional domains of aquaporin-1: Keys to physiology, and targets for drug discovery. Curr. Pharm. Des. 2007, 13, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Dorward, H.S.; Du, A.; Bruhn, M.A.; Wrin, J.; Pei, J.V.; Evdokiou, A.; Price, T.J.; Yool, A.J.; Hardingham, J.E. Pharmacological blockade of aquaporin-1 water channel by AqB013 restricts migration and invasiveness of colon cancer cells and prevents endothelial tube formation in vitro. J. Exp. Clin. Cancer Res. 2016, 35, 36. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.; Saadoun, S.; Verkman, A. Aquaporins and cell migration. Pflüg. Arch 2008, 456, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Verkman, A.; Hu, J.; Verkman, A. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006, 20, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Wragg, J.W.; Heath, V.L.; Bicknell, R. Sunitinib treatment enhances metastasis of innately drug resistant breast tumors. Cancer Res. 2016, 77, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef]
- Agre, P.; Preston, G.M.; Smith, B.L.; Jung, J.S.; Raina, S.; Moon, C.; Guggino, W.B.; Nielsen, S. Aquaporin CHIP: The archetypal molecular water channel. Am. J. Physiol. Ren. Physiol. 1993, 265, F463–F476. [Google Scholar] [CrossRef]
- Yool, A.J.; Campbell, E.M. Structure, function and translational relevance of aquaporin dual water and ion channels. Mol. Asp. Med. 2012, 33, 553–561. [Google Scholar] [CrossRef]
- De Ieso, M.L.; Yool, A.J. Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis. Front. Chem. 2018, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Birdsell, D.N.; Yool, A.J. The activity of human aquaporin 1 as a cGMP-gated cation channel is regulated by tyrosine phosphorylation in the carboxyl terminal domain. Mol. Pharmacol. 2011, 81, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Baetz, N.W.; Stamer, W.D.; Yool, A.J. Stimulation of aquaporin-mediated fluid transport by cyclic GMP in human retinal pigment epithelium in vitro. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Zhang, H.; Shao, Y.; Liu, X.; Yang, L.; Huang, Y.; Fu, L.; Gu, F.; Ma, Y. Expression of aquaporin1, a water channel protein, in cytoplasm is negatively correlated with prognosis of breast cancer patients. Oncotarget 2016, 7, 8143. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.-B.; Shi, S.; Zhang, R.-J.; Wang, T.-T.; Tan, Y.-J.; Zhang, D.; Fei, X.-Y.; Ding, G.-L.; Gao, Q.; Chen, C. Aquaporin-1 plays a crucial role in estrogen-induced tubulogenesis of vascular endothelial cells. J. Clin. Endocrinol. Metab. 2013, 98, E672–E682. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Jain, R.K.; Witwer, B.; Brown, D. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc. Res. 1999, 58, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786. [Google Scholar] [CrossRef]
- Esteva-Font, C.; Jin, B.-J.; Verkman, A. Aquaporin-1 gene deletion reduces breast tumor growth and lung metastasis in tumor-producing MMTV-PyVT mice. FASEB J. 2014, 28, 1446–1453. [Google Scholar] [CrossRef]
- Mobasheri, A.; Shakibaei, M.; Marples, D. Immunohistochemical localization of aquaporin 10 in the apical membranes of the human ileum: A potential pathway for luminal water and small solute absorption. Histochem. Cell Biol. 2004, 121, 463–471. [Google Scholar] [CrossRef]
- Mobasheri, A.; Airley, R.; Hewitt, S.M.; Marples, D. Heterogeneous expression of the aquaporin 1 (AQP1) water channel in tumors of the prostate, breast, ovary, colon and lung: A study using high density multiple human tumor tissue microarrays. Int. J. Oncol. 2005, 26, 1149–1158. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, T.; Luo, L.; Zhao, H.; Cheng, J.; Xiang, J.; Zhao, C. Aquaporins in human breast cancer: Identification and involvement in carcinogenesis of breast cancer. J. Surg. Oncol. 2012, 106, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Otterbach, F.; Callies, R.; Adamzik, M.; Kimmig, R.; Siffert, W.; Schmid, K.W.; Bankfalvi, A. Aquaporin 1 (AQP1) expression is a novel characteristic feature of a particularly aggressive subgroup of basal-like breast carcinomas. Breast Cancer Res. Treat. 2010, 120, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, F.; Ma, Y.; Gu, F. Cytoplasmic expression of aquaporin-1 in breast cancer cells and its relationship with clinicopathological characteristics and prognosis. Zhonghua Zhong Liu Za Zhi 2013, 35, 904–909. [Google Scholar] [PubMed]
- Kanaoka, M. Metabolism of ginseng saponins, ginsenosides, by human intestinal bacteria. J. Trad. Med. 1994, 11, 241–245. [Google Scholar]
- Karikura, M.; Miyase, T.; Tanizawa, H.; Taniyama, T.; Takino, Y. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VII. Comparison of the decomposition modes of ginsenoside-Rb1 and-Rb2 in the digestive tract of rats. Chem. Pharm. Bull. 1991, 39, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, E.; Kim, D.; Lee, J.; Yoo, J.; Koh, B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J. Ethnopharmacol. 2009, 122, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Zhao, Y.; Chen, P.; Huang, H.; Liu, H.; Jiang, H.; Zhang, R.; Wang, H. Structure-activity relationship and substrate-dependent phenomena in effects of ginsenosides on activities of drug-metabolizing P450 enzymes. PLoS ONE 2008, 3, e2697. [Google Scholar] [CrossRef]
- Bae, E.-A.; Han, M.J.; Choo, M.-K.; Park, S.-Y.; Kim, D.-H. Metabolism of 20 (S)-and 20 (R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol. Pharm. Bull. 2002, 25, 58–63. [Google Scholar] [CrossRef]
- Lu, J.-M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef]
- Li, K.; Chen, X.; Xu, J.; Li, X.; Zhong, D. Liquid chromatography/tandem mass spectrometry for pharmacokinetic studies of 20 (R)-ginsenoside Rg3 in dog. Rapid Commun. Mass Spectrom. 2005, 19, 813–817. [Google Scholar] [CrossRef]
- Qian, T.; Cai, Z.; Wong, R.N.; Mak, N.K.; Jiang, Z.-H. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J. Chromatogr. B 2005, 816, 223–232. [Google Scholar] [CrossRef]
- Cai, Z.; Qian, T.; Wong, R.N.; Jiang, Z.-H. Liquid chromatography–electrospray ionization mass spectrometry for metabolism and pharmacokinetic studies of ginsenoside Rg3. Anal. Chim. Acta 2003, 492, 283–293. [Google Scholar] [CrossRef]
- Xie, H.-T.; Wang, G.-J.; Sun, J.-G.; Tucker, I.; Zhao, X.-C.; Xie, Y.-Y.; Li, H.; Jiang, X.-L.; Wang, R.; Xu, M.-J. High performance liquid chromatographic–mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J. Chromatogr. B 2005, 818, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zheng, X.; Jiang, J.; Zhou, H.; Hu, P. Determination of ginsenoside Rg3 in human plasma and urine by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2010, 878, 2266–2273. [Google Scholar] [CrossRef]
- Huan, P.; Hailin, W.; Li, F.; Chengye, S. Pharmacokinetics of 20 (R)-Ginsenoside Rg3 in Human Volunteers. JCPS 2001, 10, 140–143. [Google Scholar]
- Bae, S.H.; Park, J.B.; Zheng, Y.F.; Jang, M.J.; Kim, S.O.; Kim, J.Y.; Yoo, Y.H.; Yoon, K.D.; Oh, E.; Bae, S.K. Pharmacokinetics and tissue distribution of ginsenoside Rh2 and Rg3 epimers after oral administration of BST204, a purified ginseng dry extract, in rats. Xenobiotica 2014, 44, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zou, H.; Kong, L.; Zhang, Y.; Pang, H.; Su, C.; Liu, G.; Hui, M.; Fu, L. Determination of ginsenoside Rg3 in plasma by solid-phase extraction and high-performance liquid chromatography for pharmacokinetic study. J. Chromatogr. B Biomed. Sci. Appl. 1999, 731, 403–409. [Google Scholar] [CrossRef]
- Kim, H.-G.; Yoo, S.-R.; Park, H.-J.; Lee, N.-H.; Shin, J.-W.; Sathyanath, R.; Cho, J.-H.; Son, C.-G. Antioxidant effects of Panax ginseng CA Meyer in healthy subjects: A randomized, placebo-controlled clinical trial. Food Chem. Toxicol. 2011, 49, 2229–2235. [Google Scholar] [CrossRef]
- Seo, S.K.; Hong, Y.; Yun, B.H.; Chon, S.J.; Jung, Y.S.; Park, J.H.; Cho, S.; Choi, Y.S.; Lee, B.S. Antioxidative effects of Korean red ginseng in postmenopausal women: A double-blind randomized controlled trial. J. Ethnopharmacol. 2014, 154, 753–757. [Google Scholar] [CrossRef]
- Lee, N.-H.; Yoo, S.-R.; Kim, H.-G.; Cho, J.-H.; Son, C.G. Safety and tolerability of Panax ginseng root extract: A randomized, placebo-controlled, clinical trial in healthy Korean volunteers. J. Altern. Complement. Med. 2012, 18, 1061–1069. [Google Scholar] [CrossRef]
- Lu, P.; Su, W.; Miao, Z.-H.; Niu, H.-R.; Liu, J.; Hua, Q.-L. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chin. J. Integr. Med. 2008, 14, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Niu, K.; Chen, X.; Xia, L.; Lu, D.; Kong, R.; Chen, Z.; Duan, Y.; Sun, J. Clinical benefit from EGFR-TKI plus ginsenoside Rg3 in patients with advanced non-small cell lung cancer harboring EGFR active mutation. Oncotarget 2016, 7, 70535. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yan, Z.; Liu, R.; Shi, P.; Qian, S.; Qu, X.; Zhu, L.; Zhang, W.; Wang, J. Prospective study of transcatheter arterial chemoembolization (TACE) with ginsenoside Rg3 versus TACE alone for the treatment of patients with advanced hepatocellular carcinoma. Radiology 2016, 280, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, G.; Rak, J.; Ruth, J.H. Inflammatory Mediators of Angiogenesis. Med. Inflamm. 2013, 2013, 610543. [Google Scholar] [CrossRef] [PubMed]
- Naldini, A.; Carraro, F. Role of inflammatory mediators in angiogenesis. Curr. Drug Targets Inflamm. Allergy 2005, 4, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Jiang, H.; Zhu, X.; Liu, X.; Li, J. Ginsenoside Rg3 promotes cytotoxicity of Paclitaxel through inhibiting NF-κB signaling and regulating Bax/Bcl-2 expression on triple-negative breast cancer. Biomed. Pharmacother. 2017, 89, 227–232. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).