Abstract
Background: Commiphora gileadensis (Hebrew: apharsemon) has been used since Biblical times to treat various ailments, and is used today in the traditional medicine of some Middle Eastern cultures. Methods: The essential oils from the stem bark, leaves, and fruits of Commiphora gileadensis—collected at the Ein Gedi Botanical Garden, Israel—were obtained by hydrodistillation and analyzed by gas chromatography–mass spectrometry. In addition, the enantiomeric distributions of the monoterpenoids in the essential oils have been determined by chiral gas chromatography. Results: The essential oils were dominated by monoterpene hydrocarbons, followed by oxygenated monoterpenoids. The major components in C. gileadensis oils were the monoterpenes α-pinene (11.1–18.4%), sabinene (15.8–35.9%), β-pinene (5.8–18.0%), p-cymene (4.8–8.4%), limonene (1.3–6.2%), γ-terpinene (0.7–8.1%), and terpinen-4-ol (5.3–18.5%). The (–)-enantiomers predominated for α-pinene, sabinene, β-pinene, limonene, and terpinen-4-ol. Conclusions: The chemical compositions of the C. gileadensis essential oils from Israel are markedly different from previously reported samples, which were rich in sesquiterpenoids. Likewise, the enantiomeric distribution of monoterpenoids is very different from Boswellia spp. essential oils.
1. Introduction
Commiphora gileadensis (L.) C. Chr. (syn. C. opobalsamum (L.) Engl.) is an medium-sized aromatic shrub native to the Red Sea area, including Saudi Arabia, Yemen, Oman, Eritrea, Ethiopia, Egypt, and Kenya [1]. In 1763, the renowned scholar and botanist Carl Linnaeus received a specimen collected in Yemen from his student Peter Forsskål, and identified it as the Biblical “Balm of Gilead” [2,3,4]. Today, there is widespread agreement among scholars that C. gileadensis is one of the myrrh species of the Burseraceae and the source of the legendary “Balm of Judea” (“Balsam”) perfume.
C. gileadensis is also known in Hebrew as “apharsemon” (5). The plant is indigenous to the land of Israel and emits a thick aromatic sap, from which the renowned perfume was made. Various historical sources point out that apharsemon was cultivated intensively in the Jordan Valley and Ein Gedi, in the western Dead Sea basin as a crop, and its perfume was admired and traded at a high price during Biblical times, during the time of the Second Jewish Temple, and during the Roman and Byzantine eras [5,6,7]. Archeological excavations around the Ein Gedi oasis in Israel, on the north-west shore of the Dead Sea, unraveled furnace-like structures, jars, and other objects dating from the sixth century B.C.E. that were comparable with those found in perfume-making workshops. This supports the historical references. In the 1990s, C. gileadensis was reintroduced to Israel and is currently cultivated in Ein Gedi, near the Dead Sea [8], renewing a long lost part of Jewish history in the land of Israel.
C. gileadensis has been used historically to treat a wide array of ailments [9,10,11,12,13], and is used today in the traditional medicine practices of some cultures in the Middle East. In Yemen and Oman, the bark exudate is used externally to treat skin disorders such as burns, wounds, and infections [6,14,15]. Among the Arab populations in the Middle East, a decoction of the aerial parts of the plant is administered as a pain reliever, a diuretic, and a laxative [16,17]. In 2010, Iluz et al. [6] investigated the medicinal properties of C. gileadensis and demonstrated that its sap had an inhibitory effect against Bacillus cereus and was capable of blocking lectins of Pseudomonas aeruginosa. This confirms the historical sources that indicated the antiseptic properties of the balsam’s sap. In 2012, Amiel et al. [18] further supported this finding by showing that (E)-caryophyllene was a key component in C. gileadensis essential oil, that the balsam’s stem extracts acted against cancerous tumor cells, and had apoptosis-inducing properties that acted selectively, eradicating tumor cells, but not healthy cells.
Although C. gileadensis has been an important medicinal plant throughout history, information about the essential oil composition of wild C. gileadensis is lacking. Therefore, in this work, the essential oil compositions attained from the stem bark, leaves, and fruits of wild C. gileadensis collected in Ein Gedi, Israel were determined and compared to the composition of commercially used C. gileadensis oil. In addition, to further characterize the essential oils, the enantiomeric distributions of the monoterpenoids in C. gileadensis essential oils were determined.
2. Materials and Methods
2.1. Plant Material
Leaves, stems, and fruits of C. gileadensis were collected at the Ein Gedi Botanical Garden. The plant was identified and collected by Nativ Dudai. Plant parts were placed within a Clevenger-type apparatus for 1.5 h and the essential oil was hydro-distilled. The commercial samples were produced by a steam distillation apparatus and supplied by the grower Mr. Guy Erlich from his plantation at Almog (near the Dead Sea), Israel.
2.2. Gas Chromatography–Mass Spectrometry
The essential oil compositions of wild and commercial C. gileadensis were analyzed by GC-MS using a Shimadzu GCMS-QP2010 Ultra (Shimadzu Scientific Instruments, Columbia, MD, USA) operated in the electron impact (EI) mode (electron energy = 70 eV) with a scan range of 40–400 amu and a scan rate of 3.0 scans/s, and GC-MS solution software. The GC column was a ZB-5 fused silica capillary column (Phenomenex, Torrance, CA, USA), 30 m length × 0.25 mm inner diameter, with a (5% phenyl)-polymethylsiloxane stationary phase and a film thickness of 0.25 μm. The carrier gas was helium, with a column head pressure of 552 kPa and flow rate of 1.37 mL/min. Injector temperature was 250 °C and the ion source temperature was 200 °C. The GC oven temperature program was set for an initial temperature of 50 °C and increased at a rate of 2 °C/min to 260 °C. A 5% w/v solution of the sample in CH2Cl2 was prepared, and 0.1 µL was injected with a splitting mode of 30:1. Identification of the oil components was based on retention indices determined by reference to a homologous series of n-alkanes, and by comparison of their mass spectral fragmentation patterns with those reported in the literature [19] and stored in our in-house MS library. Percentages were determined based on total ion current integrations of the peak areas, and are uncorrected.
2.3. Chiral Gas Chromatography–Mass Spectrometry
Chiral analysis of the essential oils of wild and commercial C. gileadensis was performed on a Shimadzu GCMS-QP2010S (Shimadzu Scientific Instruments, Columbia, MD, USA) operated in the EI mode (electron energy = 70 eV) with a scan range of 40–400 amu and a scan rate of 3.0 scans/s The GC was equipped with a Restek B-Dex 325 capillary column (Restek Corp., Bellefonte, PA, USA) (30 m × 0.25 mm ID × 0.25 μm film). The oven temperature began at 50 °C, and was then gradually raised to 120 °C at a rate of 1.5 °C/min. After reaching 120 °C, the oven temperature was increased to 200 °C at 2 °C/min intervals and kept at this temperature for 5 min. Helium was the carrier gas, and the flow rate was maintained at 1.8 mL/min. Samples were diluted to 3% w/v with CH2Cl2, and a 0.1 µL sample was injected in a split mode at a split ratio of 1:45. The monoterpenoid enantiomers were identified by comparison of retention times with authentic samples obtained from Sigma-Aldrich (Milwaukee, WI, USA). Relative enantiomer percentages were determined based on peak areas.
3. Results and Discussion
The essential oil compositions of C. gileadensis are compiled in Table 1. The essential oils were dominated by monoterpene hydrocarbons, followed by oxygenated monoterpenoids. The major components in C. gileadensis oils were the monoterpenes α-pinene (11.1–18.4%), sabinene (15.8–35.9%), β-pinene (5.8–18.0%), p-cymene (4.8–8.4%), limonene (1.3–6.2%), γ-terpinene (0.7–8.1%), and terpinen-4-ol (5.3–18.5%). Sesquiterpenoid concentrations were very low in these samples, and constituted only 0.8%, 10.6%, and 2.4% in the stem, leaf, and fruit oils, respectively. A representative gas chromatogram is shown in Figure 1.

Table 1.
Chemical compositions of the essential oils of wild and commercial Commiphora gileadensis.
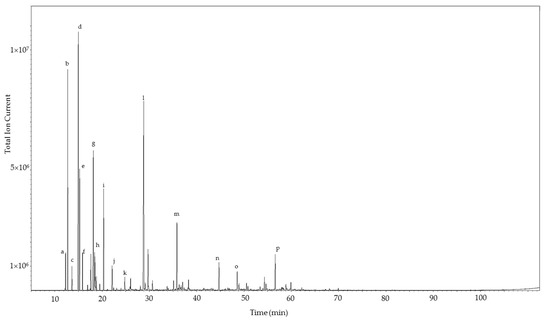
Figure 1.
Gas chromatogram of the leaf essential oil of Commiphora gileadensis. The major components are indicated: a, α-thujene; b, α-pinene; c, camphene; d, sabinene; e, β-pinene; f, myrcene; g, p-cymene; h, limonene; i, γ-terpinene; j, terpinolene; k, cis-p-menth-2-en-1-ol; l, terpinen-4-ol; m, bornyl acetate; n, (E)-caryophyllene; o, germacrene D; p, 1,10-di-epi-cubenol.
A previous examination of C. gileadensis oils from aerial parts and flowering tops collected in Makkah, Saudi Arabia showed a complete absence of monoterpene hydrocarbons, but there were high concentrations of terpinen-4-ol (8.5% and 9.8%), and of sesquiterpenoids: δ-cadinene (5.0% and 4.8%), α-calacorene (9.4% and 3.8%), viridiflorol (4.6% and 4.4%), τ-muurolol (4.5% and 3.5%), and cadalene (4.3% and 5.4%) [20]. Another investigation of C. gileadensis oil from leaves and fruits from Ein Gedi, Israel showed high concentrations of α-pinene (7.2%) and sabinene (21.1%), and richness in sesquiterpenes (E)-caryophyllene (20.1%) and germacrene D (19.6%) [18]. The high concentrations of sesquiterpenes in these earlier studies are in marked contrast to the C. gileadensis oils examined in the present study, suggesting a wide variation of essential oil compositions within the population of this plant species.
A preponderance of sesquiterpenoids over monoterpenoids in members of Commiphora has been previously shown for C. kua [21] and C. habessinica [22], where monoterpenoids have not been detected); and for C. parvifolia [23], where monoterpene hydrocarbons and oxygenated monoterpenoids were present at a level of 16%. On the other hand, C. tenuis from Ethiopia was rich in monoterpene hydrocarbons with 60.8% α-pinene, 8.8% β-pinene, 8.9% α-thujene, 6.3% sabinene, and 5.5% limonene, but had only trace amounts of sesquiterpenoids [24].
Commiphora leptophloeos leaf essential oil had nearly equal concentrations of monoterpenes and sesquiterpenes with α-phellandrene (26.3%), β-phellandrene (12.9%), (E)-caryophyllene (18.0%), α-humulene (5.5%), and germacrene D (5.6%) predominating [25]. In contrast, C. ornifolia essential oil showed no monoterpene hydrocarbons and only 4.7% sesquiterpene hydrocarbons; the bulk of the essential oil composition was made up of oxygenated monoterpenoids (56.3%) and oxygenated sesquiterpenoids (16.4%) [23].
Although the C. gileadensis essential oils were dominated by monoterpenoids, several of these have demonstrated antimicrobial activity, validating the use of exudates from this plant to treat burns, wounds, and infections. Thus, for example, α-pinene [26,27], sabinene [28], β-pinene [29], p-cymene [30,31], limonene [29], γ-terpinene [32], and terpinen-4-ol [32,33] have shown antimicrobial activities.
The enantiomeric distributions of the monoterpenoids in C. gileadensis essential oils are summarized in Table 2. The (−)-enantiomers predominated in α-thujene, α-pinene, sabinene, β-pinene, limonene, terpinen-4-ol, and α-terpineol. Borneol and bornyl acetate showed exclusively the (−)-enantiomers, but (+)-linalool and (+)-linalyl acetate predominated over the (−)-enantiomers. The chiral gas chromatogram of the leaf oil is shown in Figure 2. Currently, there is little information about the enantiomeric distribution of terpenoids in members of the Burseraceae, and apparently none regarding Commiphora spp. However, Boswellia species have been examined (Table 2) [34,35,36]. Boswellia carterii essential oil from Somalia also showed predominantly the (−)-enantiomers in α-thujene, α-pinene, sabinene, β-pinene, and limonene [35]. Basar and co-workers found that (+)-α-thujene dominates in B. carterii from Ethiopia [34]. In contrast, the (+)-enantiomers dominated in α-thujene, α-pinene, sabinene, and β-pinene in B. sacra essential oil from Oman [35]. On the other hand, analysis of a Boswellia sp. from Somalia showed (−)-α-thujene, (+)-α-pinene, (+)-sabinene, (+)-limonene, and (+)-terpinen-4-ol predominating [36]. (−)-Bornyl acetate was observed in the B. carterii sample from Ethiopia [34].

Table 2.
Enantiomeric distribution [(+):(−)] of monoterpenoids from Commiphora gileadensis and Boswellia species essential oils.
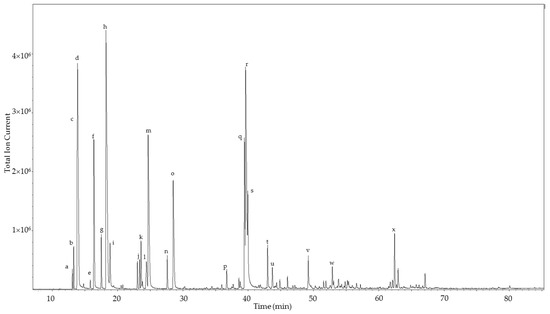
Figure 2.
Chiral gas chromatogram of the leaf essential oil of Commiphora gileadensis. The major components are indicated: a, (+)-α-thujene; b, (−)-α-thujene; c, (+)-α-pinene; d, (−)-α-pinene; e, (+)-β-pinene; f, (−)-β-pinene; g, (+)-sabinene; h, (−)-sabinene; i, myrcene; j, (+)-limonene; k, α-terpinene; l, (−)-limonene; m, p-cymene; n, terpinolene; o, γ-terpinene; p, (−)-borneol; q, (+)-terpinen-4-ol; r, (−)-terpinen-4-ol; s, (−)-bornyl acetate; t, (−)-α-terpineol; u, (+)-α-terpineol; v, (E)-caryophyllene; w, germacrene D; x, 1,10-di-epi-cubenol.
Chiral gas chromatography has been used as an analytical technique to detect outliers from authenticated oils and the possible adulteration of commercially important essential oils [37]. Since C. gileadensis has the potential for commercial utilization, this study serves to establish the enantiomeric distributions of the monoterpenoids in authentic unadulterated C. gileadensis essential oils. In addition, this work provides a comparison between C. gileadensis and other members of the Burseraceae (Boswellia spp.).
4. Conclusions
The chemical composition of essential oils from the stems, leaves, and fruits of Commiphora gileadensis have been shown to be rich in monoterpenoids, but a dearth of sesquiterpenoids, in contrast to previous reports of C. gileadensis essential oils; there is apparently wide variation in C. gileadensis volatiles. The enantiomeric distribution of monoterpenoids showed little variation between the different essential oil samples of C. gileadensis, contrasted markedly from Boswellia spp. essential oils.
Acknowledgment
We are grateful to Guy Erlich for providing the commercial sample of C. gileadensis essential oil.
Author Contributions
N.D. and P.S. conceived and designed the experiments; N.D., A.S. and P.S. performed the experiments; P.S. and W.N.S. analyzed the data; N.D., P.S. and W.N.S. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eslamieh, J. Commiphora gileadensis. Cactus Succul. J. 2011, 83, 206–210. [Google Scholar] [CrossRef]
- Willis, J.C. A Dictionary of the Flowering Plants and Ferns, 8th ed.; Cambridge University Press: Cambridge, UK, 1973. [Google Scholar]
- Hepper, F.N.; Fris, I. Plants of Pehr Forsskal’s Flora Aegyptiaco-Arabica; Royal Botanic Gardens: Kew, UK, 2000. [Google Scholar]
- Boulos, L. Flora of Egypt. Volume One: Azollaceae—Oxalidaceae; Al Hadara Publishing: Cairo, Egypt, 1999. [Google Scholar]
- Ben Yehoshua, S.; Rachmilevitch, S.; Amiel, E.; Ofir, R.; Dudai, N.; Soloway, E. Revival of the extinct balm of Gilead in Istrael: Studying its anti-cancer activity. Acta Hortic. 2015, 1088, 509–514. [Google Scholar] [CrossRef]
- Iluz, D.; Hoffman, M.; Gilboa-Garber, N.; Amar, Z. Medicinal properties of Commiphora gileadensis. Afr. J. Pharm. Pharmacol. 2010, 4, 516–520. [Google Scholar]
- Feliks, Y. The incese of the tabernacle. In Pomegranites & Golden Bells; Wright, D.P., Freedman, D.N., Hurvitz, A., Eds.; Eisenbrauns: Winona Lake, Indiana, 1995; pp. 125–149. [Google Scholar]
- Dudai, N.; Raz, A.; Hofesh, N.; Rozenzweig, N.; Aharon, R.; Fischer, R.; Chaimovitsh, D.; Daniel, S. Antioxidant activity and phenol content of plant germplasm originating in the Dead Sea area. Isr. J. Plant Sci. 2008, 56, 227–233. [Google Scholar] [CrossRef]
- Lev, E. Reconstructed materia medica of the Medieval and Ottoman al-Sham. J. Ethnopharmacol. 2002, 80, 167–179. [Google Scholar] [CrossRef]
- Lardos, A. The botanical materia medica of the Iatrosophikon—A collection of prescriptions from a monastery in Cyprus. J. Ethnopharmacol. 2006, 104, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Lev, E.; Amar, Z. Reconstruction of the inventory of materia medica used by members of the Jewish community of medieval Cairo according to prescriptions found in the Taylor-Schechter Genizah collection, Cambridge. J. Ethnopharmacol. 2006, 108, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Lev, E. Drugs held and sold by pharmacists of the Jewish community of medieval (11–14th centuries) Cairo according to lists of materia medica found at the Taylor-Schechter Genizah collection, Cambridge. J. Ethnopharmacol. 2007, 110, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Lev, E.; Amar, Z. “Fossils” of practical medical knowledge from medieval Cairo. J. Ethnopharmacol. 2008, 119, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Doa’a Anwar, I.; Amani, S.; Alzoreqy, A.; Al-Dbhani, A.; Al-Fteih, L.; Abu-Al-fatah, T.; QojaNazer, H.; Alnoor, E. Therapeutic and preventive effects of Commiphora gileadensis against diethylnitrosamine-induced hepatic injury in albino rats. Afr. J. Pharm. Pharmacol. 2016, 10, 356–363. [Google Scholar]
- Al-Mahbashi, H.M.; El-Shaibany, A.; Saad, F.A. Evaluation of acute toxicity and antimicrobial effects of the bark extract of Bisham (Commiphora gileadensis L.). J. Chem. Pharm. Res. 2015, 7, 810–814. [Google Scholar]
- Essawi, T.; Srour, M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000, 70, 343–349. [Google Scholar] [CrossRef]
- Wineman, E.; Douglas, I.; Wineman, V.; Sharova, K.; Jaspars, M.; Meshner, S.; Bentwich, Z.; Cohen, G.; Shtevi, A. Commiphora gileadensis sap extract induces cell cycle-dependent death in immortalized keratinocytes and human dermoid carcinoma cells. J. Herb. Med. 2015, 5, 199–206. [Google Scholar] [CrossRef]
- Amiel, E.; Ofir, R.; Dudai, N.; Soloway, E.; Rabinsky, T.; Rachmilevitch, S. β-Caryophyllene, a compound isolated from the biblical balm of gilead (Commiphora gileadensis), is a selective apoptosis inducer for tumor cell lines. Evidence-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Abbas, F.A.; Al-Massarany, S.M.; Khan, S.; Al-Howiriny, T.A.; Mossa, J.S.; Abourashed, E.A. Phytochemical and biological studies on Saudi Commiphora opobalsamum L. Nat. Prod. Res. 2007, 21, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Awadh Ali, N.A.; Wurster, M.; Arnold, N.; Lindequist, U.; Wessjohann, L. Essential oil composition from oleogum resin of Soqotraen Commiphora kua. Rec. Nat. Prod. 2008, 2, 70–75. [Google Scholar]
- Awadh Ali, N.A.; Wurster, M.; Lindequist, U. Chemical composition of essential oil from the olegum resin of Commiphora habessinica (Berg.) Engl. from Yemen. J. Essent. Oil Bear. Plants 2009, 12, 244–249. [Google Scholar]
- Mothana, R.A.; Al-Rehaily, A.J.; Schultze, W. Chemical analysis and biological activity of the essential oils of two endemic Soqotri Commiphora species. Molecules 2010, 15, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Asres, K.; Tei, A.; Moges, G.; Sporer, F.; Wink, M. Terpenoid composition of the wound-induced bark exudate of Commiphora tenuis from Ethiopia. Planta Med. 1998, 64, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.C.S.; Milet-Pinheiro, P.; Da Silva, P.C.B.; Da Silva, A.G.; Da Silva, M.V.; Navarro, D.M.; Da Silva, N.H. (E)-Caryophyllene and α-humulene: Aedes aegypti oviposition deterrents elucidated by gas chromatography-electrophysiological assay of commiphora leptophloeos leaf oil. PLoS ONE 2015, 10, e0144586. [Google Scholar] [CrossRef] [PubMed]
- Pichette, A.; Larouche, P.L.; Lebrun, M.; Legault, J. Composition and antibacterial activity of Abies balsamea essential oil. Phytother. Res. 2006, 20, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.M.; Noletto, J.A.; Vogler, B.; Setzer, W.N. Abaco bush medicine: Chemical composition of the essential oils of four aromatic medicinal plants from Abaco Island, Bahamas. J. Herb. Spices Med. Plants 2006, 12, 43–65. [Google Scholar] [CrossRef]
- Wanner, J.; Schmidt, E.; Bail, S.; Jirovetz, L.; Buchbauer, G.; Gochev, V.; Girova, T.; Atanasova, T.; Stoyanova, A. Chemical composition and antibacterial activity of selected essential oils and some of their main compounds. Nat. Prod. Commun. 2010, 5, 1359–1364. [Google Scholar] [PubMed]
- Kubo, I.; Muroi, H.; Kubo, A. Naturally occurring antiacne agents. J. Nat. Prod. 1994, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Delgado, B.; Fernandez, P.S.; Palop, A.; Periago, P.M. Effect of thymol and cymene on Bacillus cereus vegetative cells evaluated through the use of frequency distributions. Food Microbiol. 2004, 21, 327–334. [Google Scholar] [CrossRef]
- Kiskó, G.; Roller, S. Carvacrol and p-cymene inactivate Escherichia coli O157:H7 in apple juice. BMC Microbiol. 2005, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T. V Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003, 95, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Naturforsch. 2007, 62c, 507–513. [Google Scholar] [CrossRef]
- Basar, S.; Koch, A.; König, W.A. A verticillane-type diterpene from Boswellia carterii essential oil. Flavour Fragr. J. 2001, 16, 315–318. [Google Scholar] [CrossRef]
- Woolley, C.L.; Suhail, M.M.; Smith, B.L.; Boren, K.E.; Taylor, L.C.; Schreuder, M.F.; Chai, J.K.; Casabianca, H.; Haq, S.; Lin, H.K.; et al. Chemical differentiation of Boswellia sacra and Boswellia carterii essential oils by gas chromatography and chiral gas chromatography-mass spectrometry. J. Chromatogr. A 2012, 1261, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Pappas, R.S. First reporting on the chemistry and biological activity of a novel Boswellia chemotype: The methoxy alkane frankincense. Global J. Sci. Front. Res. B Chem. 2016, 16, 1–9. [Google Scholar]
- Wong, Y.F.; Davies, N.W.; Chin, S.T.; Larkman, T.; Marriott, P.J. Enantiomeric distribution of selected terpenes for authenticity assessment of Australian Melaleuca alternifolia oil. Ind. Crop. Prod. 2015, 67, 475–483. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).