Abstract
Background: The objective of this study is to find novel antineoplastic agents that display greater toxicity to malignant cells than to neoplasms. In addition, the mechanisms of action of representative compounds are sought. This report describes the cytotoxicity of a number of dimers of 3,5-bis(benzylidene)-4-piperidones against human malignant cells (promyelocytic leukemia HL-60 and squamous cell carcinoma HSC-2, HSC-3, and HSC-4). Methods: Tumor specificity was evaluated by the selectivity index (SI), that is the ratio of the mean CC50 for human non-malignant oral cells (gingival fibroblasts, pulp cells, periodontal ligament fibroblasts) to that for malignant cells. Results: The compounds were highly toxic to human malignant cells. On the other hand, these molecules were less toxic to human non-malignant cells. In particular, a potent lead molecule, 3b, was identified. A QSAR study revealed that the placement of electron-releasing and hydrophilic substituents into the aryl rings led to increases in cytotoxic potencies. The modes of action of a lead compound discovered in this study designated 3b were the activation of caspases-3 and -7, as well as causing PARP1 cleavage and G2 arrest, followed by sub-G1 accumulation in the cell cycle. This compound also depolarized the mitochondrial membrane and generated reactive oxygen species in human colon carcinoma HCT116 cells. In conclusion, this study has revealed that, in general, the compounds described in this report are tumor-selective cytotoxins.
1. Introduction
A major interest in our laboratories is the synthesis of novel α,β-unsaturated ketones, which were designed as candidate antineoplastic agents [1]. A number of these compounds react with thiols [2,3,4], which is likely an important contributor to cytotoxic effects. In particular, we are interested in 3,5-bis(benzylidene)-4-piperidones, whose general structure is displayed in Figure 1. However, the aim of the present investigation is not only to find compounds with marked antineoplastic properties but those that display greater toxicity to neoplasms than to non-malignant cells. This selective toxicity may arise since after an initial chemical insult, cancer cells may be more susceptible or more sensitive to subsequent toxic effects than non-malignant cells are [5,6]. Thus, in Series 2–4, whose structures are displayed in Figure 1, there are four sites at which sequential interactions with cellular constituents could occur.
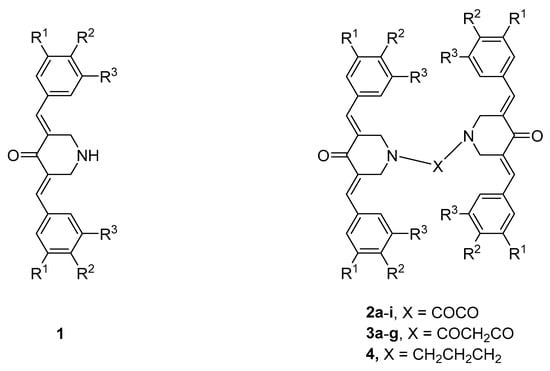
Figure 1.
Structures of the compounds in Series 1–4. The aryl substituents in Series 2 and 3 are as follows: a: R1 = R2 = R3 = H; b: R1 = R3 = H, R2 = F; c: R1 = R3 = H, R2 = Cl; d: R1 = R2 = Cl, R3 = H; e: R1 = R3 = H, R2 = CH3; f: R1 = R2 = (OCH3)2, R3 = H; g: R1 = R2 = R3 = OCH3; h: R1 = R3 = H, R2 = OCH3; i: R1 = R3 = H, R2 = N (CH3)2. In the case of Compound 4, R1 = R2 = R3 = H.
Previous studies with dimeric compounds are as follows [7]. The initial study used a diacyl linker and the compounds displayed potent cytotoxicity towards a variety of leukemic cells and lymphomas [7]. A subsequent study revealed that these compounds were cytotoxic to colon cancers, as well as leukemias [8]. An important observation was the discovery of the multidrug-resistant revertant properties of these compounds [8]. Subsequently, Series 2–4 were prepared and most of these compounds demonstrated high potency to various colon cancers, as well as leukemic and lymphoma cells [9]. However, limited evidence was provided as to whether greater toxicity for neoplastic cells was being demonstrated or not. Hence, the decision was made to evaluate the compounds in Series 2–4 against the HL-60, HSC-2, HSC-3, and HSC-4 neoplastic cell lines, as well as the HGF, HPC, and HPLF non-malignant cells. Should significant potency and especially selective toxicity be demonstrated, QSAR and mode of action investigations would be envisaged.
2. Materials and Methods
2.1. Syntheses of Compounds
The synthesis of the compounds has been described previously [7].
2.2. Cytotoxicity Assays
Human promyelocytic leukemia [HL-60 (RCB3683)] (purchased from Riken Cell Bank, Tsukuba, Japan) was cultured at 37 °C in RPMI1640 medium (Fujifilm Wako Pure Chemical Co., Osaka, Japan) supplemented with 10% heat (56 °C, 30 min) inactivated FBS (Sigma-Aldrich Inc. St. Louis, MO, USA), 100 U/mL penicillin G, and 100 µg/mL streptomycin sulfate in a humidified 5% CO2 incubator (MCO-170 AICUVD-P, Panasonic Healthcare Co., Ltd., Gunma, Japan). Human oral squamous cell carcinoma (OSCC) cell lines derived from tongue [HSC-2 (RCB1945), HSC-3 (RCB1975), HSC-4 (RCB1902)] (Riken Cell Bank), and human normal oral cells (gingival fibroblasts, HGF; pulp cell, HPC; periodontal ligament fibroblast, HPLF), established from the first premolar extracted tooth in the lower jaw and periodontal tissues of a twelve-year-old girl, according to the guidelines of Meikai University Ethic Committee (No. A0808), were cultured in DMEM medium (Fujifilm Wako Pure Chemical Co.) supplemented with 10% FBS and antibiotics. The compounds in Series 2–4 were evaluated against HL-60, HSC-2, HSC-3, HSC-4, HGF, HPC, and HPLF cells based on a literature procedure, as described previously [1]. In brief, near confluent cells were incubated in triplicate for 48 h at 37 °C with different concentrations (0, 0.039, 0.078, 0.156, 0.312, 0.625, 1.25, 2.5, 6.3, 12.5, 25, 50, 100 µM) of the compounds; then, viable cell numbers were measured using the MTT method. From the dose–response curve, a 50% cytotoxic concentration (CC50) was determined. All samples were dissolved in dimethylsulfoxide (DMSO). The toxicity of DMSO alone was calculated and subtracted. The CC50 values were determined from dose–response curves.
2.3. Activation of Caspases-3 and -7
Both 3b (0.5 µM) and 3c (0.5 µM) were examined for their ability to activate caspases-3 and -7 in HSC cells. This determination was accomplished using the Caspase-Glo 3/7 assay (Promega Corporation, Madison, WI, USA). In brief, this assay uses a tetrapeptide sequence DEVD, which is optimized for caspase and luciferase activities. The cell lysate was incubated with the Caspase-Glo reagent (buffer and substrate) for 1 h and the luminescence was measured. The resultant luminescent signal produced by luciferase was confirmed to be proportional to the amount of caspase-3 and -7 activity.
2.4. PARP1 Cleavage and Activation of Bim, Bax, Puma, and Bak
Antibodies against cleaved PARP1, bim, bax, puma, and bak were obtained from Cell Signaling Technology (Beverley, MA, USA) while the anti-actin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
The HSC-2 cells were cultured for 24 h and, then, treated with 0.5 µM of 3b or 3c for 24 h. The cells were scraped and collected in lysis buffer (purchased from Cell Signaling Technology), which contained 1 mM of phenylmethanesulfonyl fluoride and one tablet of protease inhibitor cocktail (Complete, EDTA-free; Roche Diagnostics, Mannheim, Germany). The proteins were subjected to 8% SDS-polyacrylamide gel electrophoresis and, then, transferred to a polyvinylidene difluoride membrane saturated with 5% nonfat dry milk. The membranes were incubated with rabbit monoclonal anti-cleaved PARP (Cell Signaling Technology, Beverly, MA, USA; #9541, 1:1000), or beta-actin antibody (Sigma-Aldrich, St Louis, MO, USA; A5441, 1:10,000) for 1 h at room temperature. The membranes were washed with Tris-buffered saline containing 0.1% Tween 80. And, the secondary antibodies were horseradish peroxidase-conjugated goat antimouse or antirabbit, which reacted with the chemoluminescent substrate system and were exposed to X-ray films.
2.5. Cell Cycle Analysis
The effect of 3b (0.5 µM) and 3c (0.5 µM) on the phase distribution of HSC-2 cells after 24 h of incubation was determined using the protocol of a manufacturer (BD Pharmingen, BD BioSciences, San Jose, CA, USA). In brief, treated and untreated cells were suspended at a density of ~1 × 106 cells/mL in cooled phosphate-buffered saline (PBS) without calcium and magnesium ions and fixed with 70% ethanol overnight at −20 °C. The cells were washed with cooled PBS and re-suspended in a propidium iodide/RNase staining buffer in the dark at room temperature for 0.25 h. Both acquisition and analysis were performed using EPICS ALTRA (Beckman Coulter, Brea, CA, USA) and EXPO 32 software.
2.6. Mitochondrial Membrane Potential Determination
The determination of the IC50 values of 3b, 3c, and 5-FU on the mitochondrial membrane potential in HCT 116 cells was undertaken using a literature procedure [10]. In brief, the cells were incubated at 37 °C in an atmosphere of 5% carbon dioxide for 24 h. The compounds were dissolved in DMSO, added to the cells, and incubated for 48 h. Then, a fluorescent dye, m-chlorophenylhydrazone (CCCP), and 2,4-dinitrophenol (DNP) were added to the cells, which were incubated for 0.5 h. After the addition of tetramethylrhodamine ethyl ester, the fluorescence of the cells was determined using a plate reader.
2.7. Reactive Oxygen Species Determination
The determination of the effects of 3b, 3c, and 5-FU on the generation of reactive oxygen species in HCT 116 cells was accomplished using a literature procedure [11]. In brief, the cells were incubated at 37 °C in an atmosphere of 5% carbon dioxide for 24 h. Subsequently, solutions of the compounds in DMSO were added to the cells, which were incubated for 48 h. After 2′,7′-dichlorofluorescein was added to the cells, incubation continued for another 0.5 h and the fluorescence of the cells was measured spectrophotometrically (excitation wavelength 485 nm; emission wavelength, 530 nm).
2.8. Quantitative Structure-Activity Relationships
The physicochemical constants for the aryl substituents in 2a–g and 3a–g, which were used in the QSAR determinations, were taken from the literature [12]. The linear and semilogarithmic plots were made using a commercial statistical package [13].
3. Results
The compounds in Series 2 and 3 were prepared by reacting an amount of 3,5-bis(benzylidene)-4-piperidones 1 with oxalyl chloride to produce 2a–i or with malonyl chloride to form 3a–g. In addition, a related compound, Compound 4, was prepared by reacting 1a (1, R1 = R2 = R3 = H) with 1,3-dibromopropane. These unsaturated ketones were evaluated against human promyelocytic leukemia cells (HL-60), as well as the human oral squamous cell carcinoma cell lines HSC-2, HSC-3, and HSC-4. These data are presented in Table 1 and reveal that, in general, the compounds in Series 2–4 are potent cytotoxins, which are much more toxic than the two reference compounds melphalan and curcumin. The compounds displaying the greatest average cytotoxic potencies are 2a, b, g, 3a, b, and g. In addition, these compounds were evaluated against human gingival fibroblasts (HGFs), human pulp cells (HPCs), and human periodontal ligament fibroblasts (HPLFs), which are non-malignant cells. These results are displayed in Table 2. Compounds with the highest CC50 values, i.e., those that are best tolerated by the non-malignant cells with average CC50 values in excess of 50 µM, are 2c, h, i, and 3d, as well as the drug melphalan. Selectivity index (SI) figures were generated by dividing the average CC50 values towards HGF, HPC, and HPLF cells by the CC50 value towards a neoplastic cell line. These results are displayed in Table 1. The compounds with the highest SI values are 2f and 3b, which are clearly lead molecules. A QSAR study revealed that cytotoxic potencies were correlated positively with the magnitude of the Hammett sigma (σ) values and Hansch pi (π) values of the aryl substituents. In addition, the ADME properties of seven potent cytotoxins were investigated and the result is presented in Table 3. The results reveal that future studies with analogs of Series 2–4 should have lower molecular weights and hydrophobicity than the current analogs.

Table 1.
Evaluation of the compounds in Series 2–4 against human malignant cells.

Table 2.
Evaluation of the compounds in Series 2–4 against human non-malignant cells.

Table 3.
ADME parameters of 2a, b, f, g, 3a, b, f, g, and 4.
A number of mode of action studies were undertaken with 3b and 3c using HSC-2 cells. The enone 3b induced caspases-3 and -7 (Figure 2), causing the cleavage of PARP (Figure 3) and G2M arrest in the cell cycle (Figure 4). Both compounds were evaluated for their effect on the mitochondrial membrane potential (Figure 5) and generating reactive oxygen species in HCT116 cells (Figure 6).
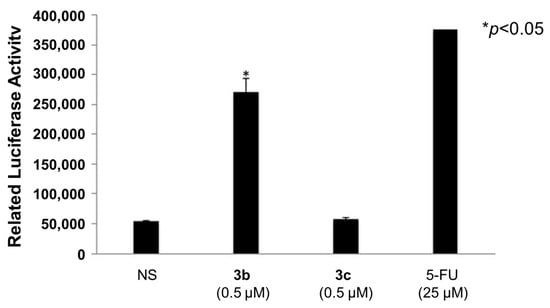
Figure 2.
Evaluation of 3b and 3c for the induction of caspases-3 and -7 in HSC-2 cells; 5-Fluorouracil (5-FU, 25 µM) was used as a positive control. Each value represents mean ± S.D. (n = 3) * p < 0.05 (statistically significant from non-stimulation, NS). The CC50 values of 3b and 3c in HSC-2 cells were 0.041 and 5.5 µM (two orders of difference); the concentration of 0.5 µM (in between) was selected for this experiment.
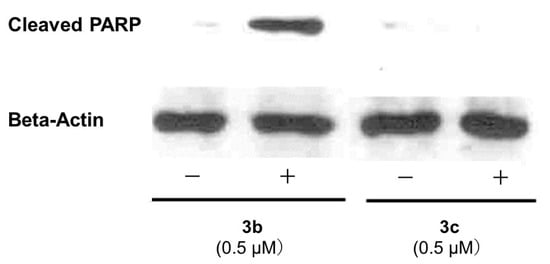
Figure 3.
Examination of 3b (0.5 µM) and 3c (0.5 µM) for the cleavage of PARP1 in HSC-2 cells.
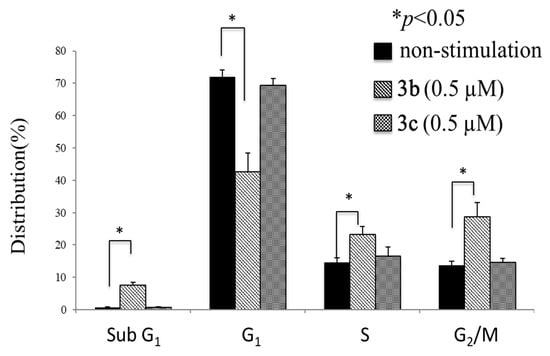
Figure 4.
Cell cycle analysis of HSC-2 cells after treatment with 3b (0.5 µM) and 3c (0.5 µM). Each value represents mean ± S.D. (n = 3) * p < 0.05 (statistically significant from non-stimulation, NS).
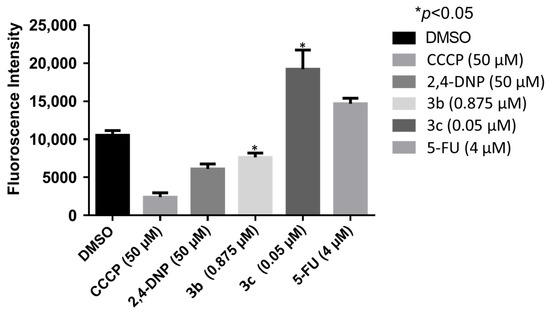
Figure 5.
The effect of 3b and 3c on the mitochondrial membrane potential of HCT116 cells. Each value represents mean ± S.D. (n = 3) * p < 0.05 (statistically significant from control, DMSO).
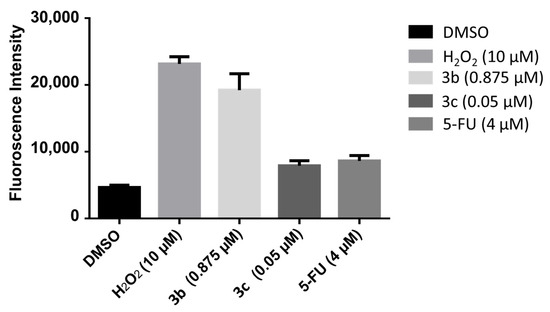
Figure 6.
The efficacy of 3b and 3c in generating reactive oxygen species in HCT116 cells. Each value represents mean ± S.D. (n = 3), p < 0.05 (statistically significant from control, DMSO).
4. Discussion
The results of evaluating 2a–i, 3a–g, and 4 against the HL60, HSC-2, HSC-3, and HSC-4 neoplastic cells are presented in Table 1. With the exception of 2i and 3d, the compounds are potent cytotoxins. No less than 53% of the CC50 values are submicromolar and, if one removes the data for the two outliers 2i and 3d, then, 60% of the CC50 values are submicromolar. Of particular note are five compounds that have CC50 values in the double-digit nanomolar range (10−8 M) in all four bioassays, namely, 2b (4-F), 2g [3,4,5-(OCH3)3], 3a (H), 3b (4-F), and 3g [3,4,5-(OCH3)3], and are lead molecules.
The importance of the spacer group between the two piperidyl nitrogen atoms was addressed. The outliers 2i and 3d (as well as 2d) were omitted from consideration. The average CC50 values of 2a–c, e–g and 3a–c, e–g are 1.62 and 1.57 µM, respectively, indicating that both linker groups may be used in developing these compounds. The average CC50 values of the three carbon spacers in 3a and 4 are 0.06 and 0.21 µM, respectively, revealing that the two oxygen atoms in the malonyl group of 3a enhance cytotoxic potencies in general.
Comparisons were made between the potencies of the compounds in Series 2–4 and established antineoplastic agents. Melphalan was chosen as a reference compound since, like conjugated enones, it is an alkylating agent. This drug has good potency towards HL-60 cells. However, it has low toxicity towards HSC-2, HSC-3, and HSC-4 cells and, thus, virtually all of the compounds in Series 2–4 are more potent than melphalan. Curcumin was selected since it is an enone and has antineoplastic properties [14]. However, in general, the biodata of Series 2–4 in Table 1 and Table 2 are markedly superior to those displayed by curcumin in terms of antineoplastic properties and SI values.
While it is important to find compounds that will kill neoplastic cells, the identification of molecules that have a substantially greater toxicity to neoplasms than to non-malignant cells is a major objective of this study. Hence the compounds in Series 2–4 were evaluated against HGF, HPC, and HPLF non-malignant cells. These data are presented in Table 2.
The data in Table 2 reveal considerable variations in the average CC50 values, which range from 0.19 µM (3g) to >100 µM (2i, 3d). Of primary interest are the compounds with high CC50 values (less toxic compounds). In this regard, compounds with average CC50 values of greater than 10 µM are 2c–f, h, and i and 3c–e, i.e., a little over half of the compounds. Particularly noteworthy are the average CC50 results for both 2c, d, and e and 3c, d, and e, which reveal that compounds with the 4-chloro-3,4-dichloro and 4-methyl aryl substituents are found in compounds with low cytotoxicity. One should note the tolerance of HGF, HPC, and HPLF cells to the reference compounds melphalan and curcumin.
Under clinical conditions, tumors are surrounded by non-malignant cells. Thus, in order to examine whether greater toxicity was displayed by the tumors than by normal cells, selectivity index (SI) figures were computed. These SI values were obtained by dividing the average CC50 values towards HGF, HPC, and HPLF non-malignant cells by the CC50 figures towards a specific cancer cell line. The SI values are presented in Table 1. The following observations may be made. Virtually all of the SI values recorded in Table 1 are greater than 1, providing evidence of the ability of the compounds in Series 2–4 to display tumor-selective toxicity. The average SI values of the compounds in Series 2–4 are greater than 10 in the case of 2a–c, e, f, and g, as well as in 3a, b, e, and 4, i.e., in 59% of the cases. It is of interest to note that there are twice as many compounds in Series 2 than those that are found in Series 3, which have SI values greater than 10. In particular, the average SI figures for 2f (57.0) and 3b (133) identify them as being important lead molecules.
In order to obtain some guidelines regarding expanding the project in the future, a quantitative structure-activity relationship (QSAR) approach was undertaken in regard to both potency and selectivity. In the case of potency, the electronic, hydrophilic, and steric properties of the aryl substituents in 2a–g and 3a–g are influenced by the magnitude of the Hammett σ, Hansch π, and molar refractivity (MR) constants of the aryl substituents. Linear plots were made between the CC50 values of 2a–g in the HL-60 cells and, first, the σ values, then, the π constants, and, subsequently, the MR values.
This process was repeated using the CC50 figures of 2a–g towards HSC-2, HSC-3, and HSC-4 cells. Then these determinations were repeated, except the CC50 values of 3a–g, not 2a–g, were used. All of these determinations were repeated, except semilogarithmic plots instead of linear ones were made.
The results obtained are presented in Tables S1 (data of 2a–g) and S2 (results of 3a–g) in the Supplemental section of this report. The following observations and conclusions were drawn:
- (1)
- A statistically significant positive relationship between the CC50 figures of 2a–g and the sigma (σ) values was obtained in a little over half of the observations made. This result reveals that the electronic properties of the aryl substituents play a significant albeit minor role in determining the magnitude of the CC50 values. The correlation is positive, revealing that the CC50 values rise (potency diminishes) as the sigma (σ) values increase in magnitude;
- (2)
- The data in Table S1 reveal clearly the positive correlation between the CC50 values and the π constants. Thus, potency increases as the hydrophilicity of the molecules falls;
- (3)
- There is no correlation between the CC50 values of 2a–g and the MR values of the aryl substituents;
- (4)
- The results in Table S2 for 3a–g reveal a similar pattern as displayed by 2a–g. There is a statistically significant positive correlation between the sigma (σ) values and the CC50 data in half of the determinations. The importance of the π values, but not the MR constants, in controlling potencies is clearly demonstrated. Thus, in planning the expansion in this series of compounds, groups should be placed in the aryl rings, which are either electron-donating, hydrophilic, or have both of these properties. Examples of such substituents are the 4-hydroxy, 4-amino, and 4-methoxy substituents with sigma (σ) values of −0.37, −0.66, and −0.27, respectively ([12], p. 49). Regarding the hydrophilic groups, the cyano, carboxylic acid, and methylsulfonyloxy have π values of −0.57, −0.32, and −0.88, respectively ([12], p. 49).
A further issue to consider is whether the magnitude of the selective toxicity is influenced by the electronic, hydrophobic, and steric properties of the groups in the aryl rings. Consequently, linear and semilogarithmic plots were made between the average SI values of 2a–g and the σ, π, and MR constants in the aryl rings of these compounds. A similar procedure was undertaken with 3a–c, e–g (3d does not have a specific average SI figure). The results of this analysis are presented in Supplementary Table S3. In the case of Series 2, the semilogarithmic plots revealed a negative correlation between the σ and π values. No other correlations were noted (p > 0.05).
Two highly important features of a candidate antineoplastic agent are their potency and SI value. In order to find lead compounds that combine both of these properties, potency-selectivity expression (PSE) figures for the compounds in Series 2–4 were obtained. The values are the products of the reciprocal of the average CC50 value against neoplastic cells and the average SI figure times 100. These data are presented in Table 2. The following compounds have PSE figures in excess of 100, namely, 2a, b, f, g, 3a, b, g, and 4. The 4-fluoro analogs 2b and 3b have the highest PSE values and are lead molecules. The PSE values of the unsubstituted compounds 3a and 4 are 290 and 120, respectively, indicating the contribution of the oxygen atoms in the spacer group of 3a to its greater PSE value than 4. One may also note that virtually all of the compounds in Series 2–4 have PSE figures far higher than those displayed by melphalan and curcumin.
An effort was made to find out if representative compounds in Series 2 and 3 possess drug-like properties. Accordingly, three compounds in both Series 2 and 3, namely, 2a, b, and g and 3a, b, and g, which have the same aryl substituents, were considered as they have good PSE figures. The rule of five articulated by Lipinski et al. [15] pertains to the size of the molecular weights, LogP values, and the numbers of hydrogen bond acceptor and donor atoms while Veber et al. [16] suggest that the number of rotatable bonds and the total polar surface (TPSA) are important physicochemical parameters. The results are portrayed in Table 3. In general, the compounds have favorable properties in terms of hydrogen bonding, rotatable bonds, and TPSA. However, the size and hydrophobicity of these molecules suggest that smaller molecules should be created with lower hydrophobic properties. The modes of action of these compounds were addressed, initially in general terms and then with specific targets in mind. As mentioned previously, a number of investigations revealed that conjugated enones react readily with thiols [2,3,4]. Specifically, various enones react only with thiol groups, even when other functionalities, such as amino and hydroxyl groups, are present [17].
Some of the biochemical mechanisms whereby an important lead compound, 3b, exerts its cytotoxic properties were investigated; also, a question was addressed, namely, why is 3b more potent than related analogs, such as 3c? The following investigations were undertaken using HSC-2 cells and the same concentrations of compounds. Caspases-3 and -7 are activated by both the death receptor and mitochondrial pathways, which lead to apoptosis. After 24 h of incubation, 3b, but not 3c, induced caspases-3 and -7 and this result is presented in Figure 2. Single-stranded DNA breaks can be repaired by poly(ADP-ribose)polymerase 1 (PARP1) [18] and, thus, compounds that cleave PARP1 reduce the extent of DNA repair and, therefore, may contribute to cytotoxic effects [19]. Figure 3 reveals that 3b, in contrast to 3c, leads to the cleavage of PARP1. Cytotoxic agents exert their bioactivity by interfering with the progression of the cell cycle. Cell cycle analysis of 3b and 3c reveals that 3b, but not 3c, causes a G2/M arrest in HSC-2 cells (Figure 4). The effect of 3b and 3c on pro-apoptotic proteins, such as bim, bax, puma, and bak, was examined in HSC-2 cells but no changes in the concentrations of these proteins were observed after 24 h (data not shown). Hence, the way in which 3b displays its cytotoxic properties includes the activation of caspases -3 and -7, cleavage of PARP1, and G2/M arrest, leading to producing a sub-G1 population (DNA fragments produced by activated DNases) [20] in the cell cycle. On the other hand, at the same concentration of 0.5 µM, 3c does not exert these effects, which explains, at least in part, the greater potency of 3b than 3c.
These mode of action studies were conducted using HSC-2 cells. The decision was made to investigate possible ways in which cytotoxicity is mediated using another cell line. Both 3b and 3c have been evaluated against human HCT116 colon cancer cells and possess IC50 values of 0.60 and 0.05 µM, respectively. One way in which cytotoxicity can be mediated is by lowering the mitochondrial membrane potential (MMP). The data presented in Figure 5 were generated using the mitochondriotropic dye tetramethylrhodamine ethyl ester, which is extruded from the mitochondria into the cytosol when the MMP is lowered. The results presented in Figure 5 reveal that 3b has a huge effect on lowering the MMP; whereas, 3c has no effect at the concentrations utilized. Another way in which cytotoxicity can be achieved is by increasing the concentration of reactive oxygen species (ROS) [21]. In this case, the magnitude of the fluorescence of the dye 2′,3′-dichlorofluorescein diacetate revealed that, while 3b and 3c cause an increase in the ROS concentrations, 3b has approximately twice the potency of 3c (Figure 6). One may conclude that the generation of ROS contributes to the cytotoxicity observed.
In summary, this study has led to the identification of three series of enones, 2–4, which, in general, have far greater toxicity to various neoplasms than non-malignant cells. QSAR studies revealed the importance of the electronic and hydrophobic properties of the aryl substituents. The ADME studies revealed that reducing the size and hydrophobicity of the aryl substituents should be undertaken.
5. Conclusions
The results generated in this study reveal that the majority of the compounds in Series 2 and 3 are potent cytotoxins and display greater toxicity to some neoplasms than to various non-malignant cells. From the data presented in Table 1 and Table 2, the compounds 2a, b, g, 3a, b, and g are lead molecules in terms of potency while 2f and 3b have the highest SI values. The PSE values, which take into consideration both potency and SI figures, reveal that the 4-fluoro analogs 2b and 3b have the highest PSE values and are lead molecules. A QSAR study indicated that, in order to increase potency, future studies should introduce hydrophilic, electron-donating substituents into the aryl rings. To some extent, this assessment aligns with the ADME result, which suggests that smaller hydrophilic molecules may have preferential absorption characteristics. Various mode of action studies revealed a number of ways the lead molecule 3b exerts its cytotoxic properties.
From the investigations described in this report, plans may be formulated to expand this project in different ways. For example, the preparation of analogs of 3b could include the syntheses and cytotoxic evaluation of structural isomers of 3b and changing the nature and size of the spacer group between the piperidine nitrogen atoms. Further bioevaluations could include assessing 3b and other lead compounds against other cell lines, both neoplastic and non-malignant, as well as conducting an Ames test. If these results are encouraging, metabolic and pharmacokinetic studies should be implemented.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicines11010003/s1, Table S1: Search for correlations between various physicochemical parameters of the aryl substituents in 2a–g and the CC50 values.; Table S2: Search for correlations between various physicochemical parameters of the aryl substituents in 3a–g and the CC50 values and Table S3: Search for correlations between various physicochemical parameters of the aryl substituents in 2a–g and 3a–c, e–g and the SI values.
Author Contributions
Conceptualization, J.R.D., D.K.J.G., M.K. and U.D.; methodology, S.D. and M.K.; formal analysis, J.R.D., H.S., P.K.R., M.H. and N.U.; investigation, H.S., J.R.D., S.D. and M.K.; data curation, H.S., P.K.R. and N.U.; writing—original draft preparation, J.R.D.; writing—review and editing, P.K.R. and M.H.; supervision, D.K.J.G., N.U. and U.D.; project administration, J.R.D., D.K.J.G. and U.D.; funding acquisition, J.R.D., U.D., H.S. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was supplied by the Canadian Institutes of Health Research and the Saskatchewan Health Research Foundation to J.R. Dimmock and U. Das, Japan Society for the Promotion of Science JSPS who awarded a grant-in-aid to H. Sakagami (principal investigator: No. 19592156; No. 16K11519; 20K09885); while The Office of the Executive Vice Chancellor for Academic Affairs, Indiana University Kokomo, USA provided funds for M. Hossain.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data contained within the article and Supplementary Materials.
Acknowledgments
Thanks are extended to the following funding sources for this project namely the Canadian Institutes of Health Research, the Saskatchewan Health Research Foundation, the Japanese Society for the promotion of Science and The Office of the Executive Vice Chancellor of Indiana University Kokomo. Further details are presented in the section entitled Funding.
Conflicts of Interest
The authors declare that they do not have any conflict of interest.
References
- Roayapalley, P.K.; Dimmock, J.R.; Contreras, L.; Balderrama, K.S.; Aguilera, R.J.; Sakagami, H.; Amano, S.; Sharma, R.K.; Das, U. Design, synthesis and tumour-selective toxicity of novel 1-[3-{3,5-bis(benzylidene)-4-oxo-1-piperidino}-3-oxopropyl]-4-piperidone oximes and related quaternary ammonium salts. Molecules 2021, 26, 7132. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, H.; Iranpoor, N.; Jafari, A.A. Micellar solution of sodium dodecyl sulfate (SDS) catalyzes facile Michael addition of amines and thiols to α,β-unsaturated ketones in water under neutral conditions. Adv. Synth. Catal. 2005, 147, 655–661. [Google Scholar] [CrossRef]
- Sauerland, M.; Mertes, R.; Morozzi, C.; Eggler, A.L.; Gamon, L.F.; Davies, M.J. Kinetic assessment of Michael addition reactions of alpha, beta-unsaturated carbonyl compounds to amino acid and protein thiols. Free Radic. Biol. Med. 2021, 169, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, B.; Shaygan, P. Michael addition of thiols to α,β-unsaturated carbonyl compounds under solvent-free conditions. Arkivoc 2006, xii, 130–137. [Google Scholar] [CrossRef]
- Chen, G.; Waxman, D.J. Role of cellular glutathione and glutathione S-transferase in the expression of alkylating agent cytotoxicity in human breast cancer cells. Biochem. Pharmacol. 1994, 47, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Komuro, C.; Ono, K.; Nishidai, T.; Shibamoto, Y.; Takahashi, M.; Abe, M. Chemosensitization by buthionine sulfoximine in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, U.; Varela-Ramirez, A.; Lema, C.; Aguilera, R.J.; Balzarini, J.; De Clercq, E.; Dimmock, S.G.; Gorecki, D.K.J.; Dimmock, J.R. Bis[3,5-bis(benzylidene)-4-oxo-1-piperidinyl]amides: A novel class of potent cytotoxins. ChemMedChem. 2011, 6, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Koroth, J.; Nirgude, S.; Tiwari, S.; Gopalakrishnan, V.; Mahadeva, R.; Kumar, S.; Karki, S.S.; Choudhary, B. Investigation of anti-cancer and migrastatic properties of novel curcumin derivatives on breast and ovarian cancer cell lines. BMC Complement. Altern. Med. 2019, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Vazquez, Y.; Das, S.; Das, U.; Robles-Escajeda, E.; Ortega, N.M.; Lema, C.; Varela-Ramirez, A.; Aguilera, R.J.; Balzarini, J.; De Clercq, E.; et al. Novel 3,5-bis(arylidene)-4-oxo-1-piperidinyl dimers: Structure-activity relationships and potent antileukemic and antilymphoma cytotoxicity. Eur. J. Med. Chem. 2014, 77, 315–322. [Google Scholar] [CrossRef] [PubMed]
- DiLisa, F.; Blank, P.S.; Colonna, R.; Gambassi, G.; Silverman, H.S.; Stern, H.S.; Hansford, R.G. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J. Physiol. 1995, 486, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.J. Substituent Constants for Correlation Analysis in Chemistry and Biology; John Wiley and Sons: New York, NY, USA, 1979; pp. 49–50. [Google Scholar]
- SPSS Inc. Statistical Package for Social Sciences, SPSS for Windows, Release 17.0; SPSS Inc.: Chicago, IL, USA, 2008. [Google Scholar]
- Shishodia, S.; Chaturvedi, M.M.; Agarwal, B.B. Role of curcumin in cancer therapy. Curr. Probl. Cancer. 2007, 31, 243–305. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Lahbib, K.; Aouani, I.; Abdelmelek, H.; Touil, S. Synthesis, hematological, biochemical, and neurotoxicity screening of some mannich base hydrochlorides. Toxicol. Int. 2013, 20, 268–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fisher, A.E.; Hochegger, H.; Takeda, S.; Caldecott, K.W. Poly(ADP-ribose) polymerase 1 accelerates single-strand break repair in concert with poly(ADP-ribose) glycohydrolase. Mol. Cell. Biol. 2007, 27, 5597–5605. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Martinvalet, D.; Chowdhury, D.; Zhang, D.; Schlesinger, A.; Lieberman, J. The cytotoxic T lymphocyte protease granzyme A cleaves and inactivates poly(adenosine 5′-diphosphate-ribose) polymerase-1. Blood 2009, 114, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.D.; Sørensen, C.S. The caspase-activated DNase: Apoptosis and beyond. FEBS J. 2017, 284, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Garza, K.M.; Soto, K.F.; Murr, L.E. Cytotoxicity and reactive oxygen species generation from aggregated carbon and carbonaceous nanoparticulate materials. Int. J. Nanomed. 2008, 3, 83–94. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).