Fluoroquinolone-Associated Movement Disorder: A Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analysis
2.5. Definitions
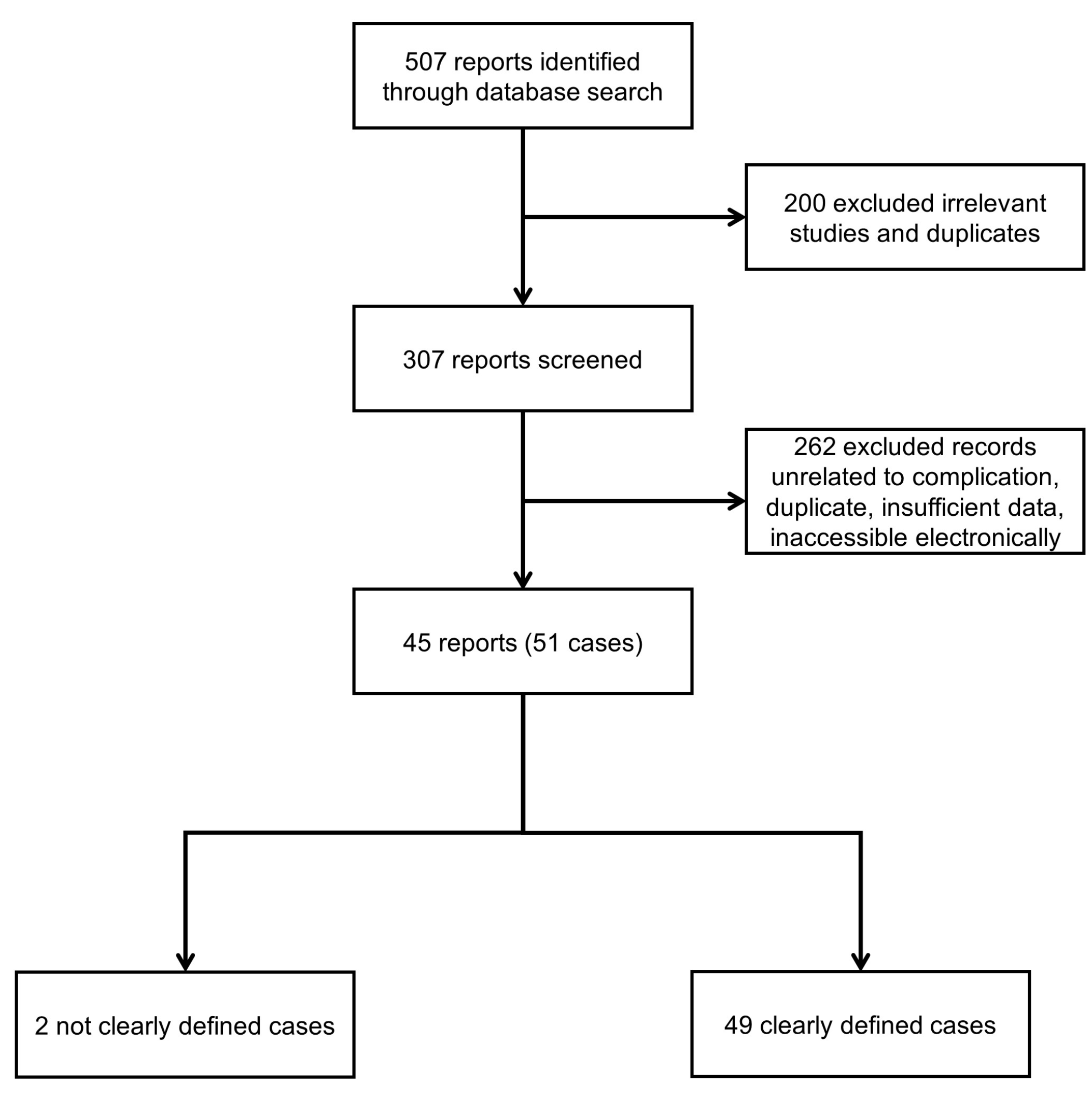
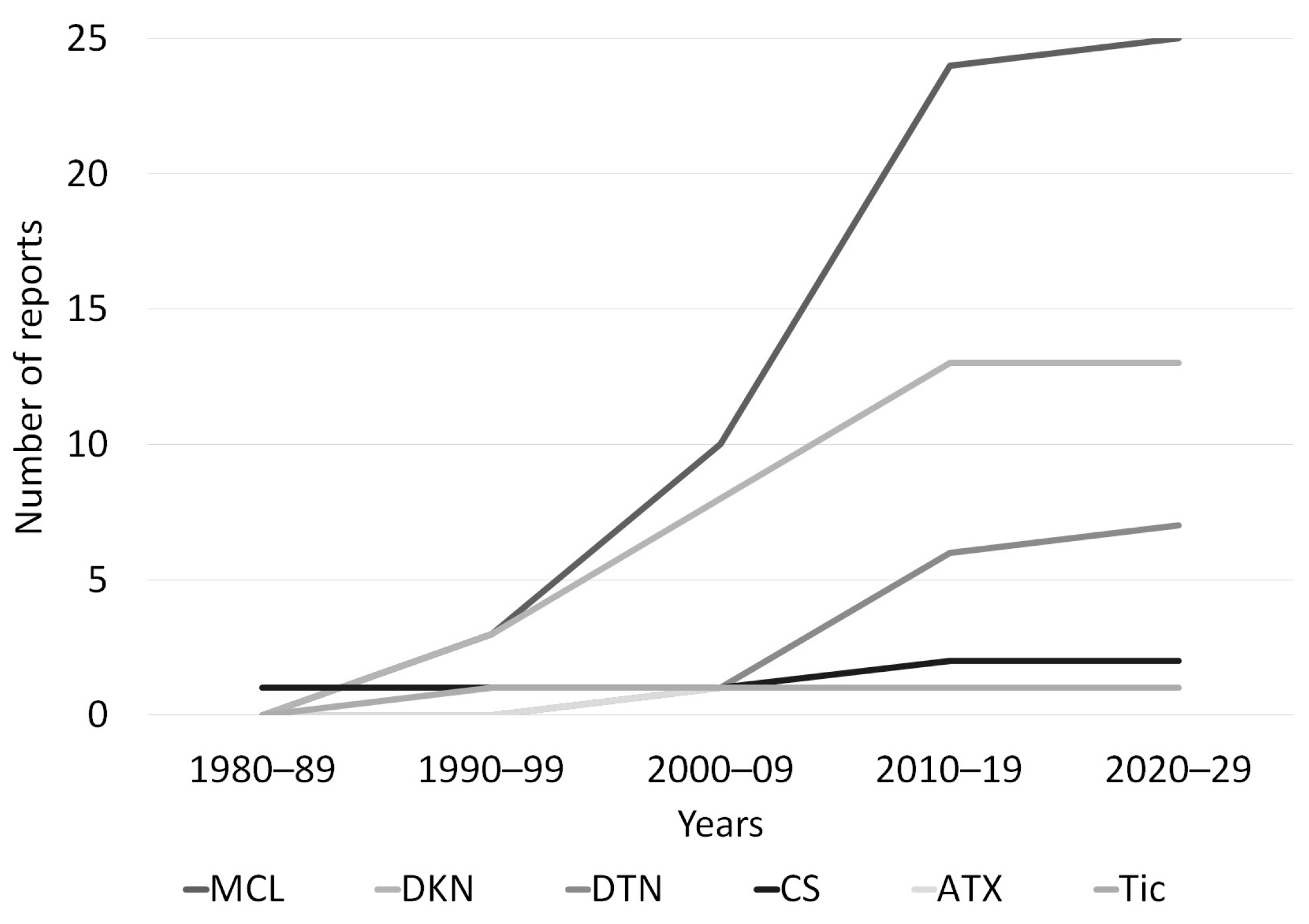
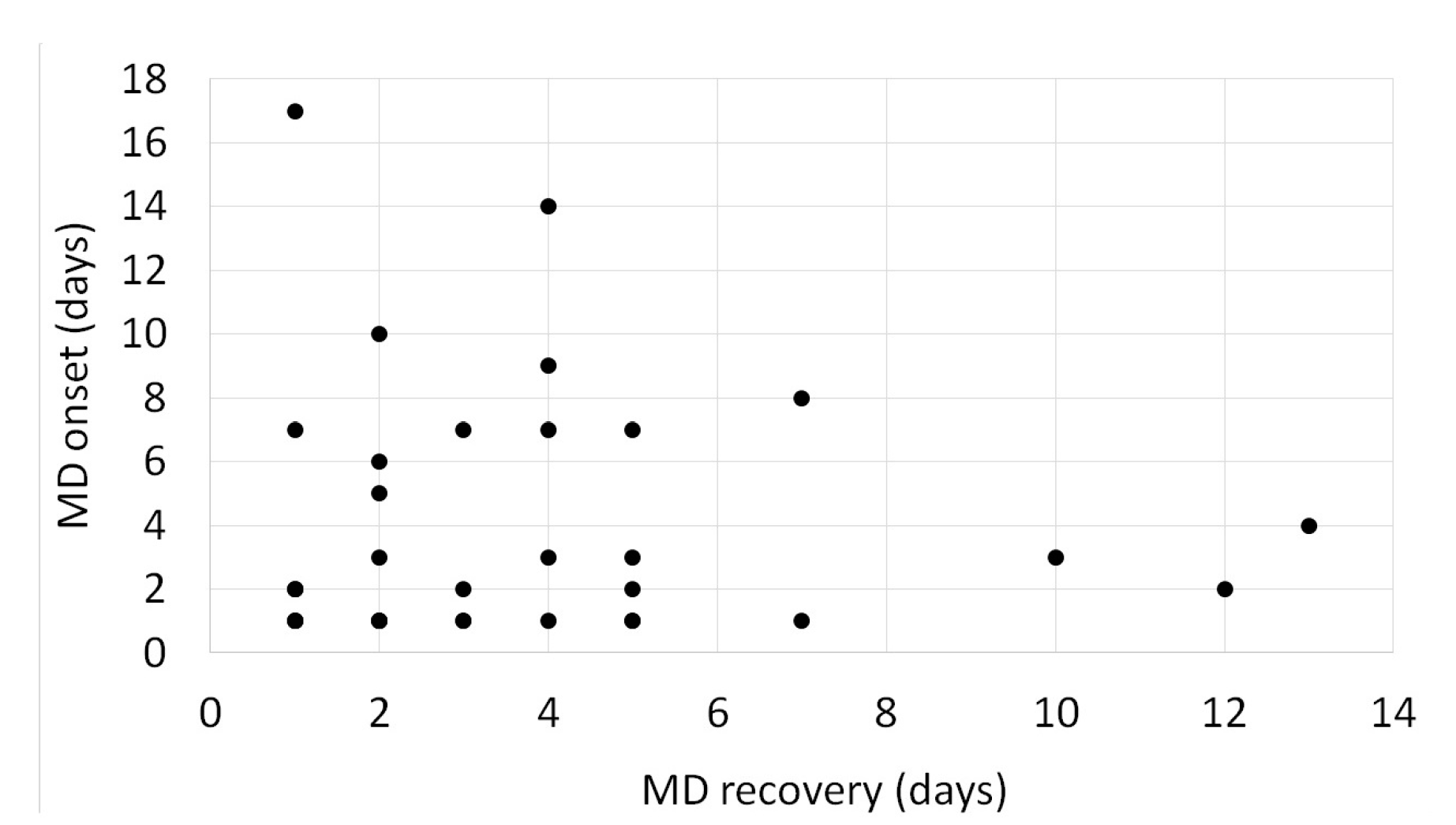
3. Results
4. Discussion
4.1. Myoclonus (MCL)–“CIPROCLONUS”
4.2. Dyskinesia (DKN)–Orofacial
4.3. Dystonia (DTN)–Rapid-Onset
4.4. Cerebellar Syndrome and Ataxia
4.5. Tics–Unique
4.6. Parkinsonism–Treatment?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kocsis, B.; Gulyás, D.; Szabó, D. Delafloxacin, Finafloxacin, and Zabofloxacin: Novel Fluoroquinolones in the Antibiotic Pipeline. Antibiotics 2021, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological Effects of Quinolones: A Family of Broad-Spectrum Antimicrobial Agents. Molecules 2021, 26, 7153. [Google Scholar] [CrossRef] [PubMed]
- López, Y.; Muñoz, L.; Gargallo-Viola, D.; Cantón, R.; Vila, J.; Zsolt, I. Uptake of Ozenoxacin and Other Quinolones in Gram-Positive Bacteria. Int. J. Mol. Sci. 2021, 22, 13363. [Google Scholar] [CrossRef] [PubMed]
- Majalekar, P.P.; Shirote, P.J. Fluoroquinolones: Blessings or Curses. Curr. Drug. Targets 2020, 21, 1354–1370. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.; Andrews, J.M.; Edwards, L.J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob. Agents Chemother. 1983, 23, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, L. The antibacterial activity of fluoroquinolone derivatives: An update (2018–2021). Eur. J. Med. Chem. 2021, 224, 113741. [Google Scholar] [CrossRef]
- Gutierrez, A.; Stokes, J.M.; Matic, I. Our Evolving Understanding of the Mechanism of Quinolones. Antibiotics 2018, 7, 32. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Azargun, R.; Gholizadeh, P.; Sadeghi, V.; Hosainzadegan, H.; Tarhriz, V.; Memar, M.Y.; Pormohammad, A.; Eyvazi, S. Molecular mechanisms associated with quinolone resistance in Enterobacteriaceae: Review and update. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 770–781. [Google Scholar] [CrossRef]
- Tomé, A.M.; Filipe, A. Quinolones: Review of psychiatric and neurological adverse reactions. Drug. Saf. 2011, 34, 465–488. [Google Scholar] [CrossRef]
- Ellis, D.E.; Hubbard, R.A.; Willis, A.W.; Zuppa, A.F.; Zaoutis, T.E.; Hennessy, S. Comparative neurological safety of fluoroquinolones versus therapeutic alternatives. Pharmacoepidemiol. Drug. Saf. 2021, 30, 797–805. [Google Scholar] [CrossRef]
- Freeman, M.Z.; Cannizzaro, D.N.; Naughton, L.F.; Bove, C. Fluoroquinolones-Associated Disability: It Is Not All in Your Head. NeuroSci 2021, 2, 235–253. [Google Scholar] [CrossRef]
- Blum, M.D.; Graham, D.J.; McCloskey, C.A. Temafloxacin syndrome: Review of 95 cases. Clin. Infect. Dis. 1994, 18, 946–950. [Google Scholar] [CrossRef]
- Althaqafi, A.; Ali, M.; Alzahrani, Y.; Ming, L.C.; Hussain, Z. How Safe are Fluoroquinolones for Diabetic Patients? A Systematic Review of Dysglycemic and Neuropathic Effects of Fluoroquinolones. Ther. Clin. Risk Manag. 2021, 17, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Gökay, S.S.; Tutun, B. Comparison of corrected QT and Tp-e/QTc interval in intoxication with drugs that cause QT prolongation in children. Güncel. Pediatri. 2020, 19, 92–99. [Google Scholar]
- Ahn, J.-H.; Jegal, H.; Choi, M.-S.; Kim, S.; Park, S.-M.; Ahn, J.; Han, H.-Y.; Cho, H.-S.; Yoon, S.; Oh, J.-H. TNFα enhances trovafloxacin-induced in vitro hepatotoxicity by inhibiting protective autophagy. Toxicol. Lett. 2021, 342, 73–84. [Google Scholar] [CrossRef]
- Baggio, D.; Ananda-Rajah, M.R. Fluoroquinolone antibiotics and adverse events. Aust. Prescr. 2021, 44, 161–164. [Google Scholar] [CrossRef]
- Pham Nguyen, T.P.; Leonard, C.E.; Bird, S.J.; Willis, A.W.; Hamedani, A.G. Pharmacosafety of fluoroquinolone and macrolide antibiotics in the clinical care of patients with myasthenia gravis. Muscle Nerve 2021, 64, 156–162. [Google Scholar] [CrossRef]
- Khaleel, A.K.; Shaari, R.B.; Nawi, M.A.; Al-Yassiri, A.M. Toxicological Aspects of Fluoroquinolones Administration: A Literature Review. Egypt. J. Chem. 2022, 65, 561–569. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H. Fluoroquinolones in the elderly: Safety considerations. Drugs. Aging 2003, 20, 289–302. [Google Scholar] [CrossRef]
- de Vries, E.; Schoonvelde, M.; Schumacher, G. No Longer Lost in Translation: Evidence that Google Translate Works for Comparative Bag-of-Words Text Applications. Politic. Anal. 2018, 26, 417–430. [Google Scholar] [CrossRef]
- Jankovic, J.; Tolosa, E. Parkinson’s Disease and Movement Disorders; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lucet, J.C.; Tilly, H.; Lerebours, G.; Gres, J.J.; Piguet, H. Neurological toxicity related to pefloxacin. J. Antimicrob. Chemother. 1988, 21, 811–812. [Google Scholar] [CrossRef]
- Schwartz, M.T.; Calvert, J.F. Potential neurologic toxicity related to ciprofloxacin. DICP 1990, 24, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Farrington, J.; Stoudemire, A.; Tierney, J. The role of ciprofloxacin in a patient with delirium due to multiple etiologies. Gen. Hosp. Psychiatry 1995, 17, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pastor, P.; Moitinho, E.; Elizalde, I.; Cirera, I.; Tolosa, E. Reversible oral-facial dyskinesia in a patient receiving ciprofloxacin hydrochloride. J. Neurol. 1996, 243, 616–617. [Google Scholar] [CrossRef]
- Thomas, R.J.; Reagan, D.R. Association of a Tourette-like syndrome with ofloxacin. Ann. Pharmacother. 1996, 30, 138–141. [Google Scholar] [CrossRef]
- Bagon, J.A. Neuropsychiatric complications following quinolone overdose in renal failure. Nephrol. Dial. Transplant 1999, 14, 1337. [Google Scholar] [CrossRef]
- Yasuda, H.; Yoshida, A.; Masuda, Y.; Fukayama, M.; Kita, Y.; Inamatsu, T. Levofloxacin-induced neurological adverse effects such as convulsion, involuntary movement (tremor, myoclonus and chorea like), visual hallucination in two elderly patients. Nihon. Ronen. Igakkai Zasshi 1999, 36, 213–217. [Google Scholar] [CrossRef]
- Lee, C.H.; Cheung, R.T.; Chan, T.M. Ciprofloxacin-induced oral facial dyskinesia in a patient with normal liver and renal function. Hosp. Med. 2000, 61, 142–143. [Google Scholar] [CrossRef]
- MacLeod, W. Case report: Severe neurologic reaction to ciprofloxacin. Can. Fam. Physician. 2001, 47, 553–555. [Google Scholar]
- Marinella, M.A. Myoclonus and generalized seizures associated with gatifloxacin treatment. Arch. Intern. Med. 2001, 161, 2261–2262. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, S.M. Reversible Encephalopathy and Delirium in Patients with Chronic Renal Failure who had Received Ciprofloxacin. Saudi. J. Kidney Dis. Transpl. 2002, 13, 163–170. [Google Scholar]
- Mohan, N.; Menon, K.; Rao, P.G. Oral gatifloxacin-induced ataxia. Am. J. Health Syst. Pharm. 2002, 59, 1894. [Google Scholar] [CrossRef] [PubMed]
- De Bleecker, J.L.; Vervaet, V.L.; De Sarro, A. Reversible orofacial dyskinesia after ofloxacin treatment. Mov. Disord. 2004, 19, 731–732. [Google Scholar] [CrossRef] [PubMed]
- Post, B.; Koelman, J.H.; Tijssen, M.A. Propriospinal myoclonus after treatment with ciprofloxacin. Mov. Disord. 2004, 19, 595–597. [Google Scholar] [CrossRef]
- Azar, S.; Ramjiani, A.; Van Gerpen, J.A. Ciprofloxacin-induced chorea. Mov. Disord. 2005, 20, 513–514. [Google Scholar] [CrossRef]
- Cheung, Y.F.; Wong, W.W.; Tang, K.W.; Chan, J.H.; Li, P.C. Ciprofloxacin-induced palatal tremor. Mov. Disord. 2007, 22, 1038–1043. [Google Scholar] [CrossRef]
- Striano, P.; Zara, F.; Coppola, A.; Ciampa, C.; Pezzella, M.; Striano, S. Epileptic myoclonus as ciprofloxacin-associated adverse effect. Mov. Disord. 2007, 22, 1675–1676. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeong, S.H.; Kim, J.W.; Lee, S.H.; Kim, J.M. A case of hemiballism as a rare side effect of ciprofloxacin in a patient with liver cirrhosis. Chemother 2009, 55, 207–210. [Google Scholar] [CrossRef]
- Sharma, D.D.; Aggarwal, A.; Sharma, R.C.; Kumar, R. A probable association of acute dystonia with gemifloxacin administration. Indian J. Med. Sci. 2009, 63, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Jayathissa, S.; Woolley, M.; Ganasegaram, M.; Holden, J.; Cu, E. Myoclonus and delirium associated with ciprofloxacin. Age Ageing 2010, 39, 762. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.O.; Machado, D.G.; Jabbari, B. Orofacial dyskinesia after moxifloxacin treatment-a case with normal hepatorenal function and review of literature. Clin. Neuropharmacol. 2012, 35, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.; Ramly, S.; Boers, P.; Casserly, L. Ciprofloxacin-associated choreoathetosis in a haemodialysis patient. BMJ Case Rep. 2013, 2013, bcr2013009293. [Google Scholar] [CrossRef]
- Anderson, R.; Chowdhury, N.; Ahmed, J.; Smalligan, R. “CIPROCLONUS”: Ciprofloxacin induced myoclonus. J. Investig. Med. 2013, 61, 390. [Google Scholar]
- Host, B.D.; Sloan, W. Orofacial dyskinesia associated with the use of levofloxacin. Ann. Pharmacother. 2014, 48, 142–144. [Google Scholar] [CrossRef]
- Kango Gopal, G.; Hewton, C.; Pazhvoor, S.K. Myoclonus associated with concomitant ciprofloxacin and oxycodone in an older patient. Br. J. Clin. Pharmacol. 2014, 77, 906–907. [Google Scholar] [CrossRef]
- Lizarraga, K.J.; Lopez, M.R.; Singer, C. Reversible craniocervical dystonia associated with levofloxacin. J. Clin. Mov. Disord. 2015, 2, 10. [Google Scholar] [CrossRef]
- Ridout, K.K.; Ridout, S.J.; Pirnie, L.F.; Puttichanda, S.P. Sudden-onset dystonia in a patient taking asenapine: Interaction between ciprofloxacin and asenapine metabolism. Am. J. Psychiatry 2015, 172, 1162–1163. [Google Scholar] [CrossRef]
- Juana, E.B.; Floristán, C.V.; Gutiérrez, A.G.; Calvo, J.I.; Juste, C.T. Myoclonus associated with Ciprofloxacin therapy. Farm. Hosp. 2016, 40, 622–623. [Google Scholar]
- Kalita, J.; Bhoi, S.K.; Betai, S.; Misra, U.K. Safety and efficacy of additional levofloxacin in tuberculous meningitis: A randomized controlled pilot study. Tuberculosis 2016, 98, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, A.A. A first case of cerebellar syndrome due to fluoroquinolone. Therapie 2017, 72, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Kayipmaz, S.; Altınöz, A.E.; Ok, N.E. Lithium Intoxication: A Possible Interaction with Moxifloxacin. Clin. Psychopharmacol. Neurosci. 2017, 15, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Kunder, S.K.; Avinash, A.; Nayak, V.; Tilak, A. A Rare Instance of Levofloxacin Induced Myoclonus. J. Clin. Diagn. Res. 2017, 11, 1–2. [Google Scholar] [CrossRef]
- van Samkar, A.; de Kleermaeker, F.; te Riele, M.G.; Verrips, A. Negative Myoclonus Induced by Ciprofloxacin. Tremor. Other Hyperkinet. Mov. 2017, 7, 500. [Google Scholar] [CrossRef]
- Bacchin, R.; Macchione, F.; Cardellini, D.; Orlandi, R.; Gajofatto, A.; Zanusso, G.; Vattemi, G. Levofloxacin-induced hemichorea-hemiballism in a patient with previous thalamic infarction. Neurol. Sci. 2018, 39, 1483–1485. [Google Scholar] [CrossRef]
- Bates, D.; Edwards, J.; Justin, C.; Fisher, M.; Switzer, A.; Morris, C. Fluoroquinolone Induced Movement Disorders: Case Report and Literature Review. Ulutas. Med. J. 2020, 4, 53–63. [Google Scholar] [CrossRef]
- Idrees, N.; Almeqdadi, M.; Balakrishnan, V.S.; Jaber, B.L. Hemodialysis for treatment of levofloxacin-induced neurotoxicity. Hemodial. Int. 2019, 23, 40–45. [Google Scholar] [CrossRef]
- Olmsted, R.; Sargsyan, Z. Levofloxacin-Induced Myoclonus. Hosp. Med. 2018, 4, 765. [Google Scholar]
- Philips, C.A.; Augustine, P. Levofloxacin associated fatal oro-facio-brachial dystonia in cirrhosis. OGH Reports 2018, 10, 12–14. [Google Scholar] [CrossRef]
- Shihabudheen, P.; Uvais, N. Multifocal dystonia induced by levofloxacin. Asian J. Pharm. Pharmacol. 2018, 4, 87–89. [Google Scholar] [CrossRef]
- Nishikubo, M.; Kanamori, M.; Nishioka, H. Levofloxacin-Associated Neurotoxicity in a Patient with a High Concentration of Levofloxacin in the Blood and Cerebrospinal Fluid. Antibiotics 2019, 8, 78. [Google Scholar] [CrossRef]
- Sugiura, M.; Shibata, K.; Saito, S.; Nishimura, Y.; Sakura, H. Levofloxacin-associated Encephalopathy with Severe Hyperventilation. Intern. Med. 2019, 58, 1495–1499. [Google Scholar] [CrossRef]
- Yildiz, M.Ç.; Arslan, M.; Gökçenoǧlu, Y.; Çalışkan, A.M.; Çalışkan, S.; Eren, İ. Dystonia as an unexpected interaction of ciprofloxacin and clozapine: A case report. Psychi. Clin. Psychopharmacol. 2019, 29, 181. [Google Scholar]
- Reddy, V.; Mittal, G.K.; Sekhar, S.; Singhdev, J.; Mishra, R. Levofloxacin-Induced Myoclonus and Encephalopathy. Ann. Indian Acad. Neurol. 2020, 23, 405–407. [Google Scholar] [PubMed]
- Chappell, K.B.; Shah, S.P.; Cutshall, B.T.; Sands, C.W. Ciprofloxacin-rasagiline drug interaction leading to dopaminergic effects. Nurse Pract. 2020, 45, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Chouksey, A.; Pandey, S. Clinical Spectrum of Drug-Induced Movement Disorders: A Study of 97 Patients. Tremor. Other Hyperkinet. 2020, 10, 48. [Google Scholar] [CrossRef]
- Kane, S. The Top 300 of 2020, ClinCalc DrugStats Database, Version 20.1; ClinCalc: Arlington Heights, IL, USA, 2022; Available online: https://clincalc com/DrugStats/Top300Drugs.aspx (accessed on 1 December 2022).
- Buehrle, D.J.; Wagener, M.M.; Clancy, C.J. Outpatient Fluoroquinolone Prescription Fills in the United States, 2014 to 2020: Assessing the Impact of Food and Drug Administration Safety Warnings. Antimicrob. Agents Chemother. 2021, 65, e0015121. [Google Scholar] [CrossRef] [PubMed]
- Sankar, A.; Swanson, K.M.; Zhou, J.; Jena, A.B.; Ross, J.S.; Shah, N.D.; Karaca-Mandic, P. Association of Fluoroquinolone Prescribing Rates with Black Box Warnings from the US Food and Drug Administration. JAMA Netw. Open 2021, 4, 2136662. [Google Scholar] [CrossRef]
- Sousa, J.; Alves, G.; Fortuna, A.; Falcão, A. Third and fourth generation fluoroquinolone antibacterials: A systematic review of safety and toxicity profiles. Curr. Drug. Saf. 2014, 9, 89–105. [Google Scholar] [CrossRef]
- Halliwell, R.F.; Davey, P.G.; Lambert, J.J. The effects of quinolones and NSAIDs upon GABA-evoked currents recorded from rat dorsal root ganglion neurones. J. Antimicrob. Chemother. 1991, 27, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ilgin, S.; Can, O.D.; Atli, O.; Ucel, U.I.; Sener, E.; Guven, I. Ciprofloxacin-induced neurotoxicity: Evaluation of possible underlying mechanisms. Toxicol. Mech. Methods 2015, 25, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A. Fluoroquinolone induced neurotoxicity: A review. J. Adv. Pharm. Edu. Res. 2013, 3, 16–19. [Google Scholar]
- Zhang, W.; Teng, M.; Yan, J.; Chen, L. Study effect and mechanism of levofloxacin on the neurotoxicity of Rana nigromaculata tadpoles exposed to imidacloprid based on the microbe-gut-brain axis. Sci. Total. Environ. 2023, 872, 162098. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, M.; Gu, H.; Zhang, L.; Shang, Y.; Wang, T.; Wang, T.; Zeng, J.; Ma, L.; Huang, W.; et al. Short-term exposure to norfloxacin induces oxidative stress, neurotoxicity and microbiota alteration in juvenile large yellow croaker Pseudosciaena crocea. Environ. Pollut. 2020, 267, 115397. [Google Scholar] [CrossRef]
- Xi, J.; Liu, J.; He, S.; Shen, W.; Wei, C.; Li, K.; Zhang, Y.; Yue, J.; Yang, Z. Effects of norfloxacin exposure on neurodevelopment of zebrafish (Danio rerio) embryos. Neurotoxicology 2019, 72, 85–94. [Google Scholar] [CrossRef]
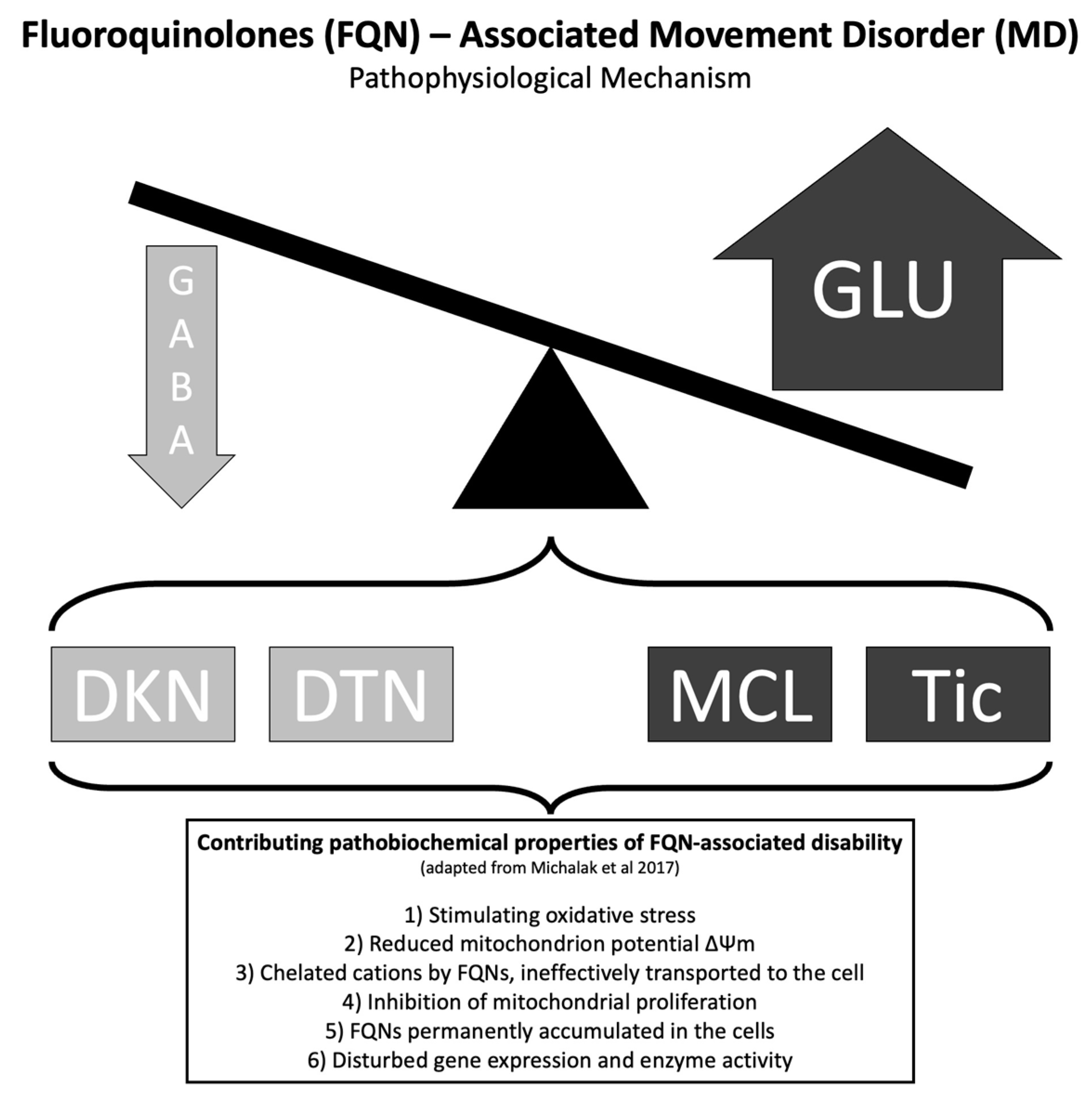
- Michalak, K.; Sobolewska-Włodarczyk, A.; Włodarczyk, M.; Sobolewska, J.; Woźniak, P.; Sobolewski, B. Treatment of the Fluoroquinolone-Associated Disability: The Pathobiochemical Implications. Oxid. Med. Cell Longev. 2017, 2017, 8023935. [Google Scholar] [CrossRef]
- Flynn, S.E.; Morrison, C.S. Neuropsychological Side Effects Associated with Fluoroquinolone Therapy: A Qualitative Review with Recommendations for Future Research. MAR Neurol. Psychol. 2022, 4, 1–14. [Google Scholar]
- Rissardo, J.P.; Caprara, A.L. Phenytoin-associated movement disorders: A literature review. Tzu Chi Med. J. 2022, 34, 409–417. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.; Durante, Í.; Rauber, A. Lithium-associated movement disorder: A literature review. Brain Circ. 2022, 8, 76–86. [Google Scholar] [CrossRef]
- Spooren, W.P.; Mulders, W.H.; Veening, J.G.; Cools, A.R. The substantia innominata complex and the peripeduncular nucleus in orofacial dyskinesia: A pharmacological and anatomical study in cats. Neurosci 1993, 52, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Alabed, S.; Latifeh, Y.; Mohammad, H.A.; Bergman, H. Gamma-aminobutyric acid agonists for antipsychotic-induced tardive dyskinesia. Cochrane Database Syst. Rev. 2018, 4, Cd000203. [Google Scholar] [CrossRef] [PubMed]
- Thaker, G.K.; Nguyen, J.A.; Strauss, M.E.; Jacobson, R.; Kaup, B.A.; Tamminga, C.A. Clonazepam treatment of tardive dyskinesia: A practical GABAmimetic strategy. Am. J. Psychiatry 1990, 147, 445–451. [Google Scholar] [PubMed]
- Nair, P.; Trisno, R.; Baghini, M.S.; Pendharkar, G.; Chung, H. Predicting Early Stage Drug Induced Parkinsonism using Unsupervised and Supervised Machine Learning. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 2020, 776–779. [Google Scholar]
- Rissardo, J.P.; Caprara, A.L. Pregabalin-associated movement disorders: A literature review. Brain Circ. 2020, 6, 96–106. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L. Buspirone-associated Movement Disorder: A Literature Review. Prague. Med. Rep. 2020, 121, 5–24. [Google Scholar] [CrossRef]
- Bulica, B.; Sidiropoulos, C.; Mahajan, A.; Zillgitt, A.; Kaminski, P.; Bowyer, S.M. Sensorimotor Integration and GABA-ergic Activity in Embouchure Dystonia: An Assessment with Magnetoencephalography. Tremor. Other Hyperkinet. Mov. 2019, 9, 1–5. [Google Scholar] [CrossRef]
- Levy, L.M.; Hallett, M. Impaired brain GABA in focal dystonia. Ann. Neurol. 2002, 51, 93–101. [Google Scholar] [CrossRef]
- Viaggi, B.; Cangialosi, A.; Langer, M.; Olivieri, C.; Gori, A.; Corona, A.; Finazzi, S.; Di Paolo, A. Tissue Penetration of Antimicrobials in Intensive Care Unit Patients: A Systematic Review&mdash—Part II. Antibiotics 2022, 11, 1193. [Google Scholar]
- Rissardo, J.P.; Caprara, A.L. Carbamazepine-, Oxcarbazepine-, Eslicarbazepine-Associated Movement Disorder: A Literature Review. Clin. Neuropharmacol. 2020, 43, 66–80. [Google Scholar] [CrossRef]
- El Ayoubi, N.; Sawaya, R.; Sawaya, R. Does Levofloxacin Improve Parkinsonian Features or Is the Improvement Only Coincidental? Clin. Neuropharmacol. 2016, 39, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, M.; Orimo, K.; Mano, T.; Miyano, R.; Sato, A.K.; Sato, K.; Ihara, R.; Hayashi, T.; Toda, T. Gait improvement after levofloxacin administration in a progressive supranuclear palsy patient. Clin. Park Relat. Disord. 2020, 3, 100080. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.H.; Takakusaki, K.; Debu, B.; Lozano, A.M.; Krishna, V.; Fasano, A.; Aziz, T.Z.; Papa, S.M.; Factor, S.A.; Hallett, M. Physiology of freezing of gait. Ann. Neurol. 2016, 80, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Ondo, W.G.; Silay, Y.S. Intravenous flumazenil for Parkinson’s disease: A single dose, double blind, placebo controlled, cross-over trial. Mov. Disord. 2006, 21, 1614–1617. [Google Scholar] [CrossRef]
- Daniele, A.; Albanese, A.; Gainotti, G.; Gregori, B.; Bartolomeo, P. Zolpidem in Parkinson’s disease. Lancet 1997, 349, 1222–1223. [Google Scholar] [CrossRef]
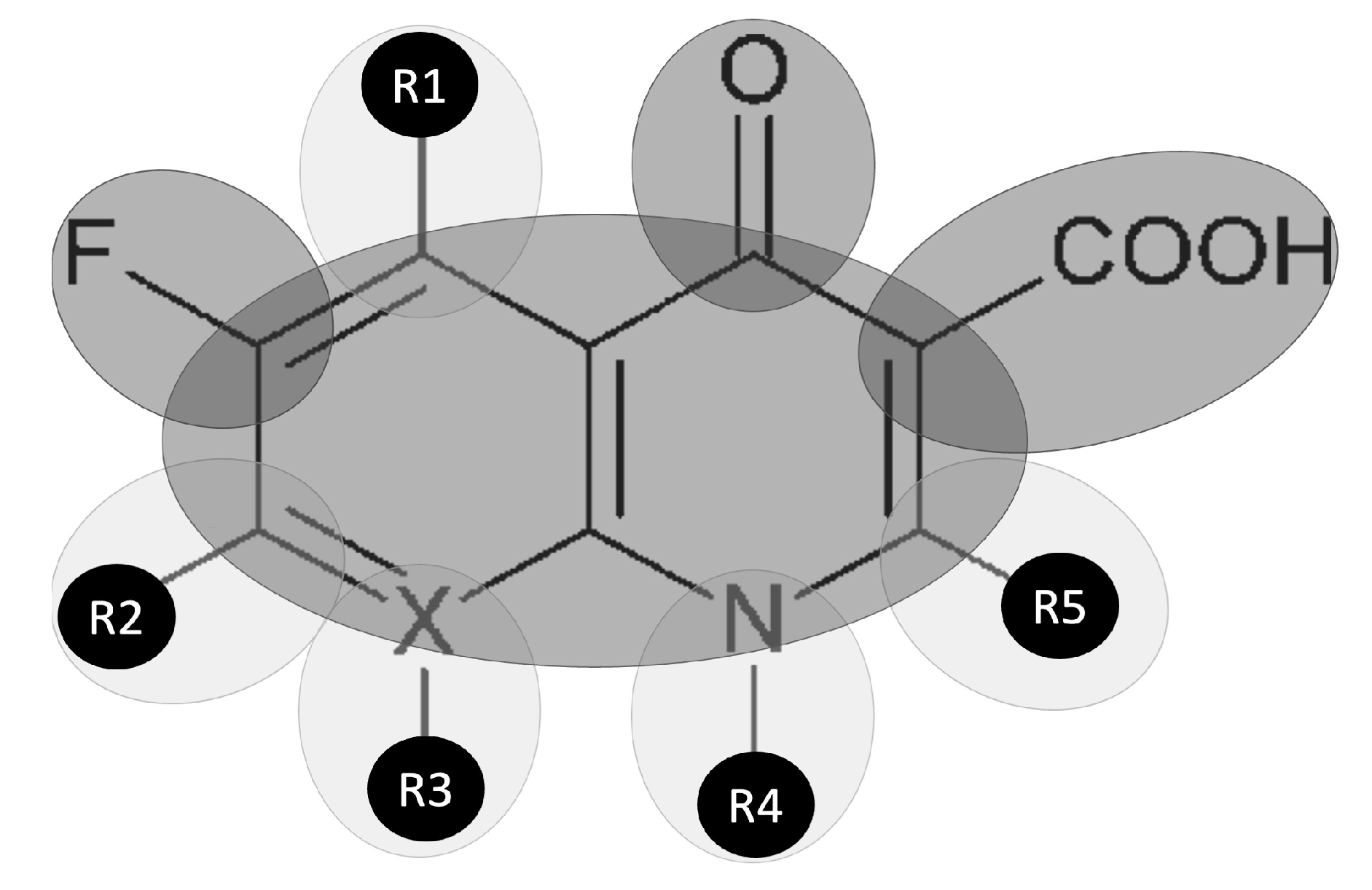
| FQN | Ciprofloxacin | Gatifloxacin | Gemifloxacin | Levofloxacin | Moxifloxacin | Ofloxacin | Pefloxacin | |
|---|---|---|---|---|---|---|---|---|
| FDA Approval | October 1987 | December 1999 | April 2003 | December 1996 | December 1999 | December 1990 | Not approved | |
| Formula | C17H18FN3O3 | C19H22FN3O4 | C18H20FN5O4 | C18H20FN3O4 | C21H24FN3O4 | C18H20FN3O4 | C17H20FN3O3 | |
| MOA | DNA gyrase | No | Yes | Yes | Yes | Yes | No | Yes |
| Topoisomerase IV | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Dosage Forms (USA approved) | Infusion solution: 200 mg/100 mL, 200 mg/20 mL, 400 mg/40 mL, 400 mg/200 mL. Oral suspension: 250 mg/5 mL, 500 mg/5 mL. Tablet: 100 mg, 250 mg, 500 mg, 750 mg. Tablet, ER: 500 mg, 1000 mg | Ophthalmic solution: 0.3%,0.5% | Tablets: 320 mg | Premix, ready-to-use injection: 250 mg/50 mL, 500 mg/100 mL, 750 mg/150 mL Oral solution: 25 mg/mL Tablet: 250 mg, 500 mg, 750 mg | Injectable solution: 400 mg/250 mL Tablet: 400 mg | Tablet: 200 mg, 300 mg, 400 mg | NA. | |
| Dosage adjustment | Renal impairment | None | Renal impairment | Renal impairment | None | Renal impairment | Renal impairment | |
| Bioavailability (%) | 50–85 | NA | 71 | 99 | 86–90 | 85–98 | 100 | |
| Peak plasma time | IR: 0.5–2 h; ER: 1–2.5 h | NA | 0.5–2 h | 1–2 h | 2 h | 1–2 h | 2 h | |
| Protein-bound (%) | 20–40 | 20 | 60–70 | 31 | 47 | 32 | 20–30 | |
| Volume of distribution | 2.1–2.7 L/kg | NA | 1.66–12.12 L/kg | 74–112 L | 1.7–2.7 L/kg | 2.4–3.5 L/kg | 100 and 140 L | |
| Metabolism | Liver | NA | Liver | Limited | Liver | Liver | Liver | |
| Half-life | 3–5 h | 7–14 h | 5–9 h | 6–8 h | PO:12 h; IV:15 h | 4–5 h | 8.6 h | |
| Elimination | Urine (30–50%) > Feces (15–43%) | NA | Feces (60%) > Urine (40%) | Urine (87%) > Feces (4%) | Urine (20%) > Feces (25%) | Urine (80%) > Feces (4%) | Urine > feces | |
| Notes | Distributed widely throughout body. | NA | Dialyzable (20–30%). Minor metabolites. | CSF concentrations about 15% of serum levels | Covers anaerobic pathogens. CYP450 not involved. | Dialyzable. | Most frequently FQN associated with tendon rupture | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rissardo, J.P.; Caprara, A.L.F. Fluoroquinolone-Associated Movement Disorder: A Literature Review. Medicines 2023, 10, 33. https://doi.org/10.3390/medicines10060033
Rissardo JP, Caprara ALF. Fluoroquinolone-Associated Movement Disorder: A Literature Review. Medicines. 2023; 10(6):33. https://doi.org/10.3390/medicines10060033
Chicago/Turabian StyleRissardo, Jamir Pitton, and Ana Letícia Fornari Caprara. 2023. "Fluoroquinolone-Associated Movement Disorder: A Literature Review" Medicines 10, no. 6: 33. https://doi.org/10.3390/medicines10060033
APA StyleRissardo, J. P., & Caprara, A. L. F. (2023). Fluoroquinolone-Associated Movement Disorder: A Literature Review. Medicines, 10(6), 33. https://doi.org/10.3390/medicines10060033








