Quercetin-Induced Enhancement of Nasal Epithelial Cells’ Ability to Produce Clara Cell 10-kD Protein In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Cell Culture
2.4. Sensitization and Challenge Procedures
2.5. Treatment of Rats with Agents
2.6. Assay for Nasal Symptoms
2.7. Preparation of Nasal Lavage Fluids
2.8. Assay for CC10
2.9. Statistical Analysis
3. Results
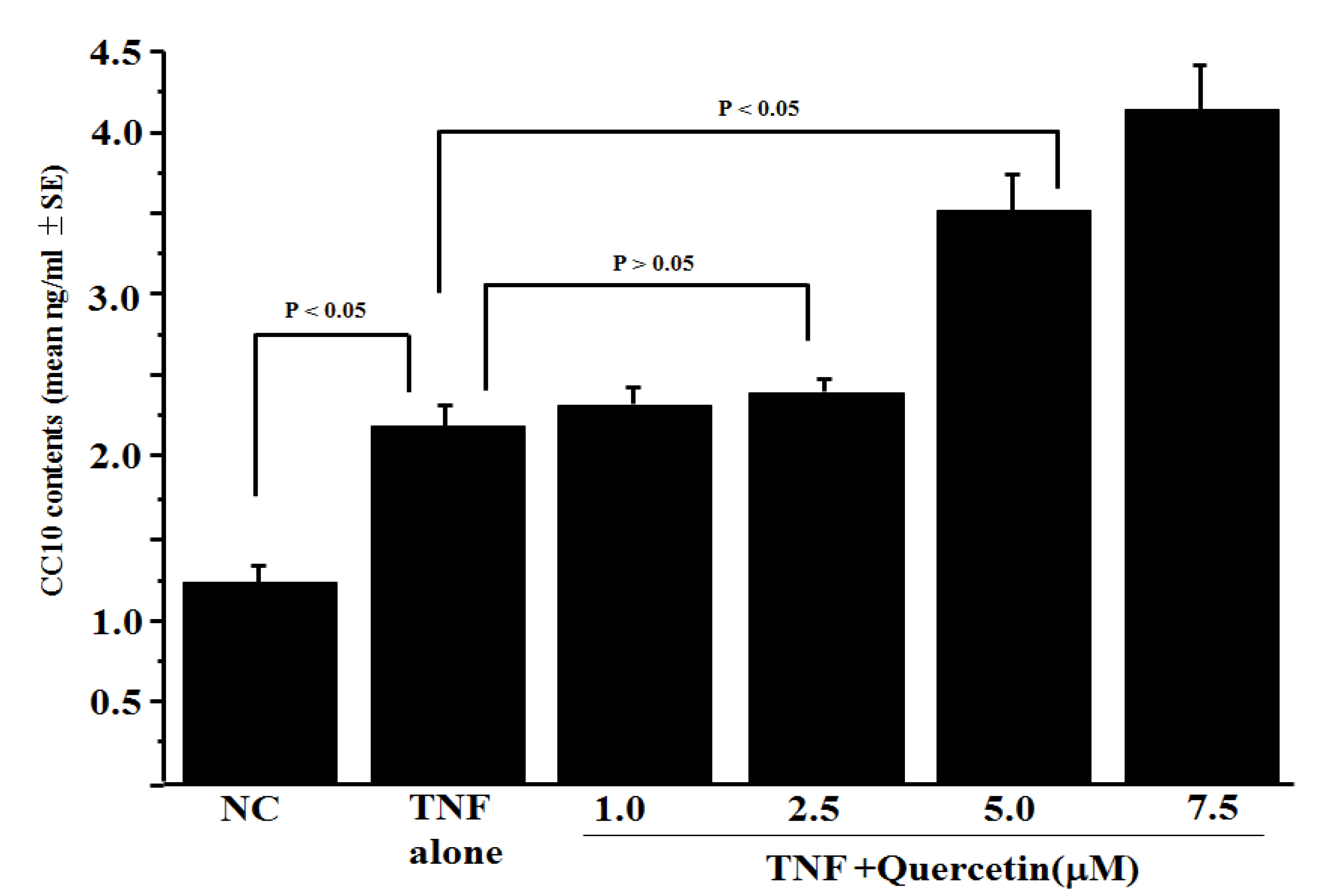
3.1. Influence of Quercetin on CC10 Production from HNEpCs In Vitro
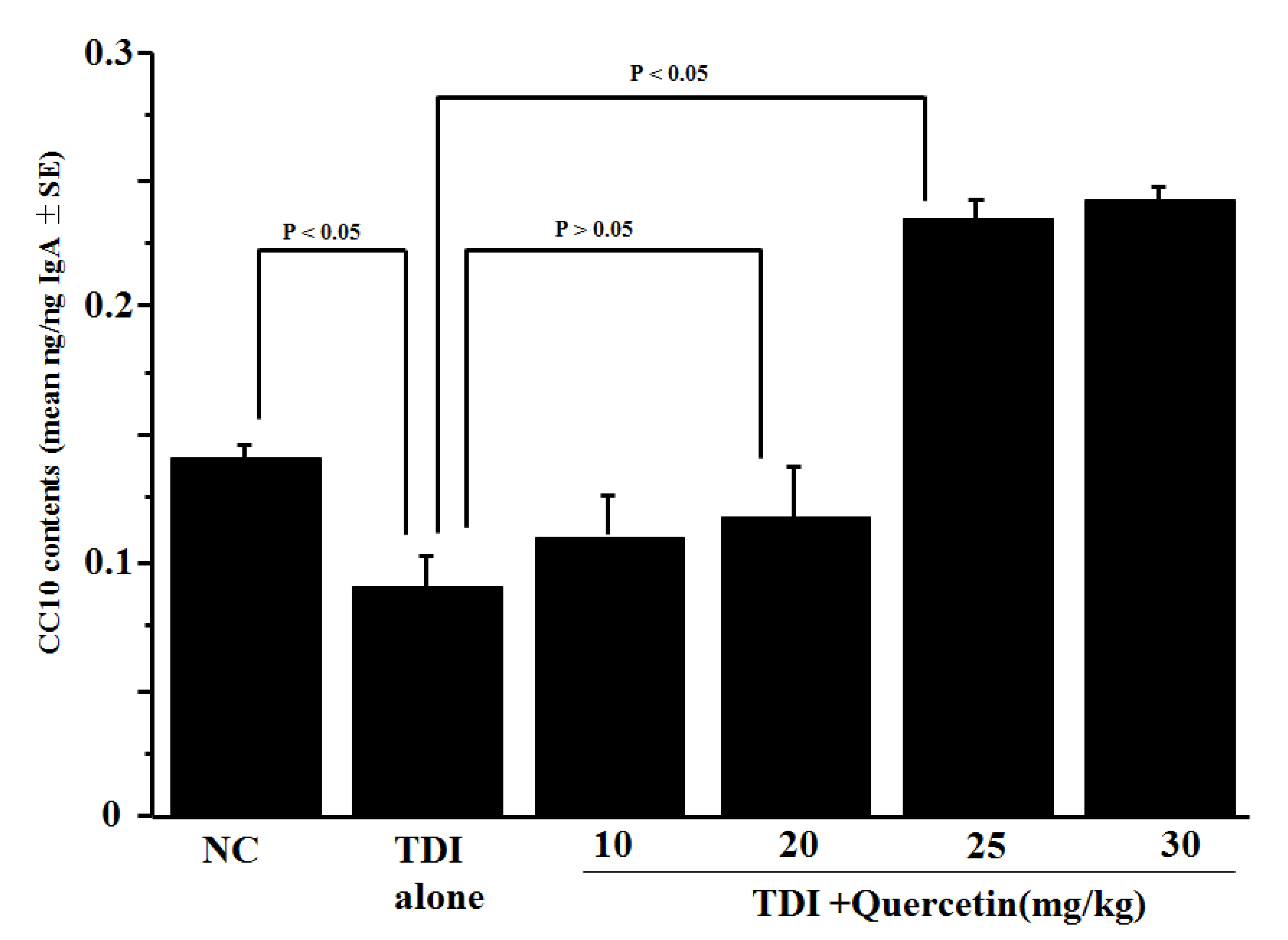
3.2. Influence of Quercetin on CC10 Production In Vivo
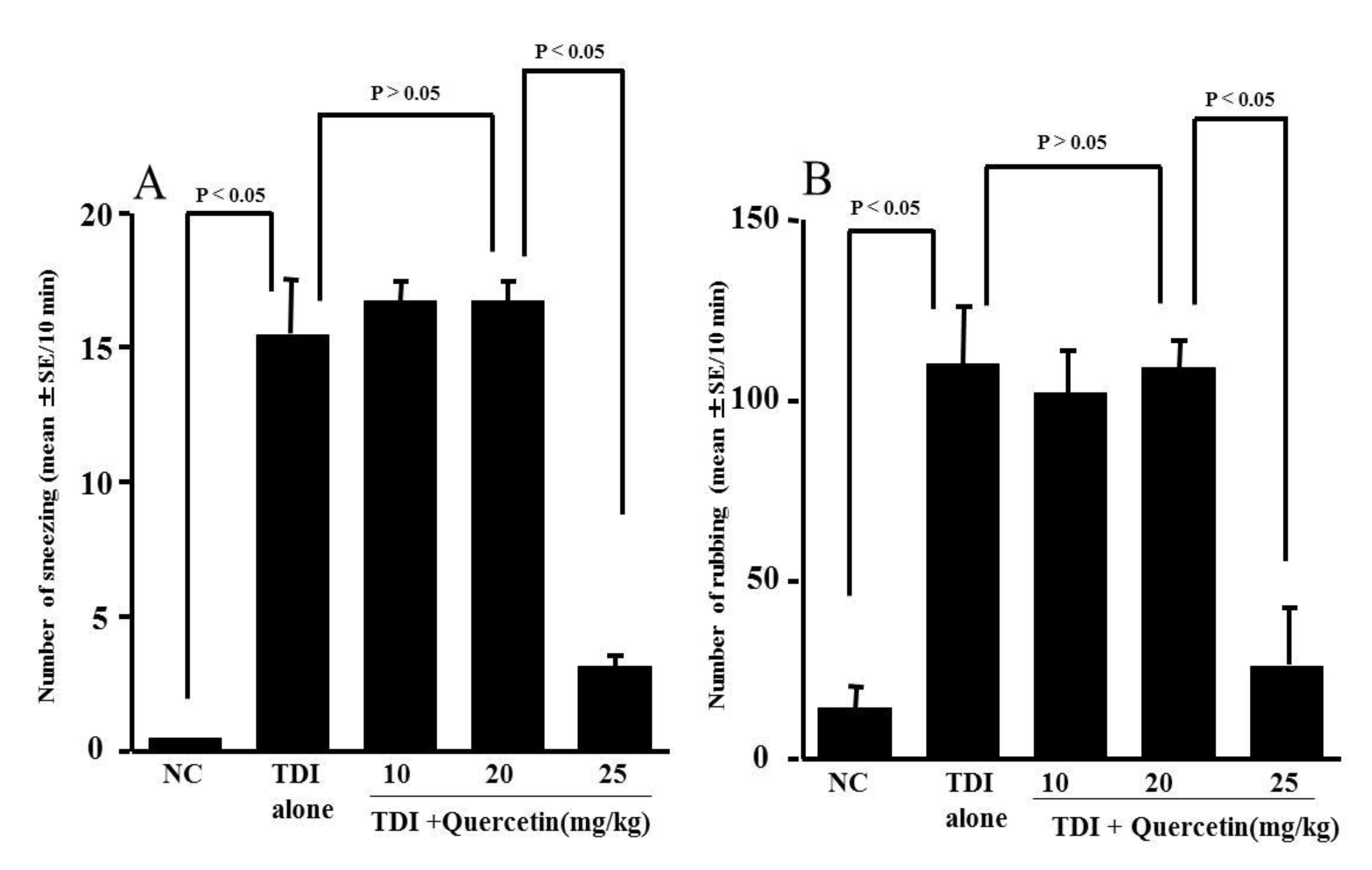
3.3. Influence of Quercetin on the Development of TDI-Induced Nasal Allergy-Like Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sur, D.K.C.; Plesa, M.L. Treatment of allergic rhinitis. Am. Fam. Physician 2015, 92, 985–992. [Google Scholar] [PubMed]
- Hoyte, F.C.L.; Nelson, H.S. Recent advance in allergic rhinitis. F1000Research 2018, 7, 1333. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Jimenez, F.; Pavon-Romero, G.; Juarez-Martinez, L.L.; Teran, L.L. Allergic rhinitis. J. Aller. Ther. 2012, S5, 007. [Google Scholar] [CrossRef]
- Derendorf, H.; Meltzer, E.O. Molecular and clinical pharmacology of intranasal corticosteroids: Clinical and therapeutic implication. Allergy 2008, 63, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Liu, Z. Clara cell 10-kD protein in inflammatory upper airway diseases. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 25–30. [Google Scholar] [CrossRef]
- Johansson, S.; Wennergren, G.; Aberg, N.; Rudin, A. Clara cell 16-kd protein downregulates T(H)2 differentiation of human naïve neonatal T cells. J. Allergy Clin. Immunol. 2007, 120, 308–314. [Google Scholar] [CrossRef]
- Long, X.; Wang, N.; Zhang, X. Role of Clara cell 10-kD protein and type 2 innate lymphoid cells in allergic rhinitis. Cell Cycle 2021, 20, 1923–1934. [Google Scholar] [CrossRef]
- Hung, C.H.; Chen, L.C.; Zhang, Z.; Chowdhury, B.; Lee, W.L.; Plunkett, B.; Chen, C.H.; Myers, A.C.; Huang, S.K. Regulation of TH2 responses by the pulmonary Clara cell secretory 10-kd protein. J. Allergy Clin. Immunol. 2004, 114, 664–670. [Google Scholar] [CrossRef]
- Almuntashiri, S.; Zhu, Y.; Han, Y.; Wang, X.; Somanath, P.R.; Zhang, D. Club cell secreted protein CC16: Potential applications in prognosis and therapy for pulmonary diseases. Clin. Med. 2020, 9, 4039. [Google Scholar] [CrossRef]
- Laucho-Contreras, M.E.; Polverino, F.; Tesfaigzi, Y.; Pilon, A.; Celli, B.R.; Owen, C.A. Club cell protein 16 (CC16) augmentation: A potential disease-modifying approach for chronic obstructive pulmonary disease (COPD). Expert Opin. Ther. Targets 2016, 20, 869–883. [Google Scholar] [CrossRef]
- Ishizawa, K.; Yoshizumi, M.; Kawai, Y.; Terao, J.; Kihira, Y.; Ikeda, Y.; Tomita, S.; Minakuchi, K.; Tsuchiya, K.; Tamaki, T. Pharmacology in health food: Metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J. Pharm. Sci. 2011, 115, 466–470. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implication for inflammation, heart disease, and cancer. Pharmacol. Review. 2000, 52, 673–751. [Google Scholar]
- Yu, Y.B.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Park, J.C. Effects of triterpenoids and flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Arch. Pharmacol. Res. 2007, 30, 820–826. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Middleton, E., Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar]
- Min, Y.D.; Choi, C.H.; Bark, H.; Son, H.Y.; Park, H.H.; Lee, S.; Park, J.W.; Park, E.K.; Shin, H.I.; Kim, S.H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and P38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef]
- Hirano, T.; Kawai, M.; Arimitsu, J.; Ogawa, M.; Kuwahara, Y.; Hagihara, K.; Shima, Y.; Narazaki, M.; Ogata, A.; Koyanagi, M.; et al. Preventive effect of a flavonoid, enzymatically modified isoquercetin on ocular symptoms of Japanese cedar pollinosis. Allergol. Int. 2009, 58, 373–382. [Google Scholar] [CrossRef]
- Kashiwabara, M.; Asano, K.; Mizuyoshi, T.; Kobayashi, H. Suppression of neuropeptide production by quercetin in allergic rhinitis model rats. BMC Comp. Altern. Med. 2016, 16, 132. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; de Silva, E.V.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercetin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef]
- Shishehbor, F.; Behroo, L.; Ghafouriyan Broujerdnia, M.; Namjoyan, F.; Latifi, S.M. Quercetin effectively quells peanuts-induced anaphylactic reactions in the peanut sensitized rats. Iran J. Allergy Asthma Immunol. 2010, 9, 27–34. [Google Scholar]
- Song, Y.; Qu, C.; Srivastava, K.; Yang, N.; Busse, P.; Zhao, W.; Li, X.M. Food allergy herbal formula 2 protection against peanut anaphylactic reaction is via inhibition of mast cells and basophils. J. Allergy Clin. Immunol. 2010, 126, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Nogaki, T.; Asano, K.; Furuta, A.; Kanai, K.; Suzaki, I.; Kanei, A.; Suzaki, H. Enhancement of Clara cell 10-kD protein (CC10) production from nasal epithelial cells by fexofenadine hydrochloride. Asian Pac. J. Allergy Immunol. 2012, 30, 139–1345. [Google Scholar] [PubMed]
- Edo, Y.; Otaki, A.; Asano, K. Quercetin enhances the thioredoxin production of nasal epithelial cells in vitro and in vivo. Medicines 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Okumo, T.; Furuta, A.; Kimura, T.; Yusa, K.; Asano, K.; Sunagawa, M. Inhibition of angiogenic factor productions by quercetin in vitro and in vivo. Medicines 2021, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Walgren, R.A.; Walle, U.K.; Walle, T. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2. Biochem. Pharmacol. 1998, 55, 1721–1727. [Google Scholar] [CrossRef]
- Hollman, P.C.; vd Gaag, M.; Mengelers, M.J.; van Trijp, J.M.; de Vries, J.H.; Katan, M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Rad. Biol. Med. 1996, 21, 703–707. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Koop, D.R. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced release of nitric oxide. Chemico-Biological Interact. 2001, 137, 43–58. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, X.; Yu, H.J.; Hua, X.Y.; Cui, Y.H.; Huang, S.K.; Liu, Z. The expression of osteoponchin and its association with Clara cell 10-kD protein in allergic rhinitis. Clin. Exp. Allergy 2010, 40, 1632–1641. [Google Scholar] [CrossRef]
- Wang, H.; Long, X.B.; Cao, P.P.; Wang, N.; Liu, Y.; Cui, Y.H.; Huang, S.K.; Liu, Z. Clara cell 10-kD protein suppresses chitinase 3-like 1 expression associated with eosinophilic chronic rhinosinusitis. Am. J. Respr. Crit. Care Med. 2010, 181, 908–916. [Google Scholar] [CrossRef]
- Wang, M.; Tang, K.; Gao, P.; Lu, Y.; Wang, S.; Wu, X. Club cell 10-kDa protein (CC10) as a surrogate for identifying type 2 asthma phenotype. J. Asthma 2022, 15, 1–9. [Google Scholar] [CrossRef]
- Jang, D.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Pasparakis, M.; Vandenabeele, P. Necrosis factor and its role in inflammation. Nature 2015, 517, 871–876. [Google Scholar] [CrossRef]
- Poynter, M.E.; Irvin, C.G.; Janssen-Heininger, Y.M.W. A prominent role of epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J. Immunol. 2003, 170, 6257–6265. [Google Scholar] [CrossRef]
- Sakai-Kashiwabara, M.; Abe, S.; Asano, K. Suppressive activity of quercetin on the production of eosinophil chemoattractants from eosinophils in vitro. In Vivo 2014, 28, 515–522. [Google Scholar]
- Weng, Z.; Zhang, B.; Asadi, S.; Sismanopoulos, N.; Butcher, A.; Fu, X.; Katsarou-Katsari, A.; Antoniou, C.; Theoharides, T.C. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in human. PLoS ONE 2012, 7, e33805. [Google Scholar] [CrossRef]
- Mukherjee, A.B.; Zhang, Z.; Chilton, B.S. Uteroglobin: A steroid-inducible immunomodulatory protein that founded the secretoglobin superfamily. Endocr. Rev. 2007, 28, 707–725. [Google Scholar] [CrossRef]
- Cui, Y.H.; Wang, Y.Y.; Liu, Z. Transdifferentiation of Clara cell 10-kDa protein secreting cells in experimental allergic rhinitis. Am. J. Rhinol. Allergy 2011, 25, 145–151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otaki, A.; Furuta, A.; Asano, K. Quercetin-Induced Enhancement of Nasal Epithelial Cells’ Ability to Produce Clara Cell 10-kD Protein In Vitro and In Vivo. Medicines 2023, 10, 28. https://doi.org/10.3390/medicines10040028
Otaki A, Furuta A, Asano K. Quercetin-Induced Enhancement of Nasal Epithelial Cells’ Ability to Produce Clara Cell 10-kD Protein In Vitro and In Vivo. Medicines. 2023; 10(4):28. https://doi.org/10.3390/medicines10040028
Chicago/Turabian StyleOtaki, Amane, Atsuko Furuta, and Kazuhito Asano. 2023. "Quercetin-Induced Enhancement of Nasal Epithelial Cells’ Ability to Produce Clara Cell 10-kD Protein In Vitro and In Vivo" Medicines 10, no. 4: 28. https://doi.org/10.3390/medicines10040028
APA StyleOtaki, A., Furuta, A., & Asano, K. (2023). Quercetin-Induced Enhancement of Nasal Epithelial Cells’ Ability to Produce Clara Cell 10-kD Protein In Vitro and In Vivo. Medicines, 10(4), 28. https://doi.org/10.3390/medicines10040028






