Novel Polyethylene Terephthalate Screw Sleeve Implant: Salvage Treatment in a Case of Spine Instability after Vertebroplasty Failure
Abstract
1. Introduction
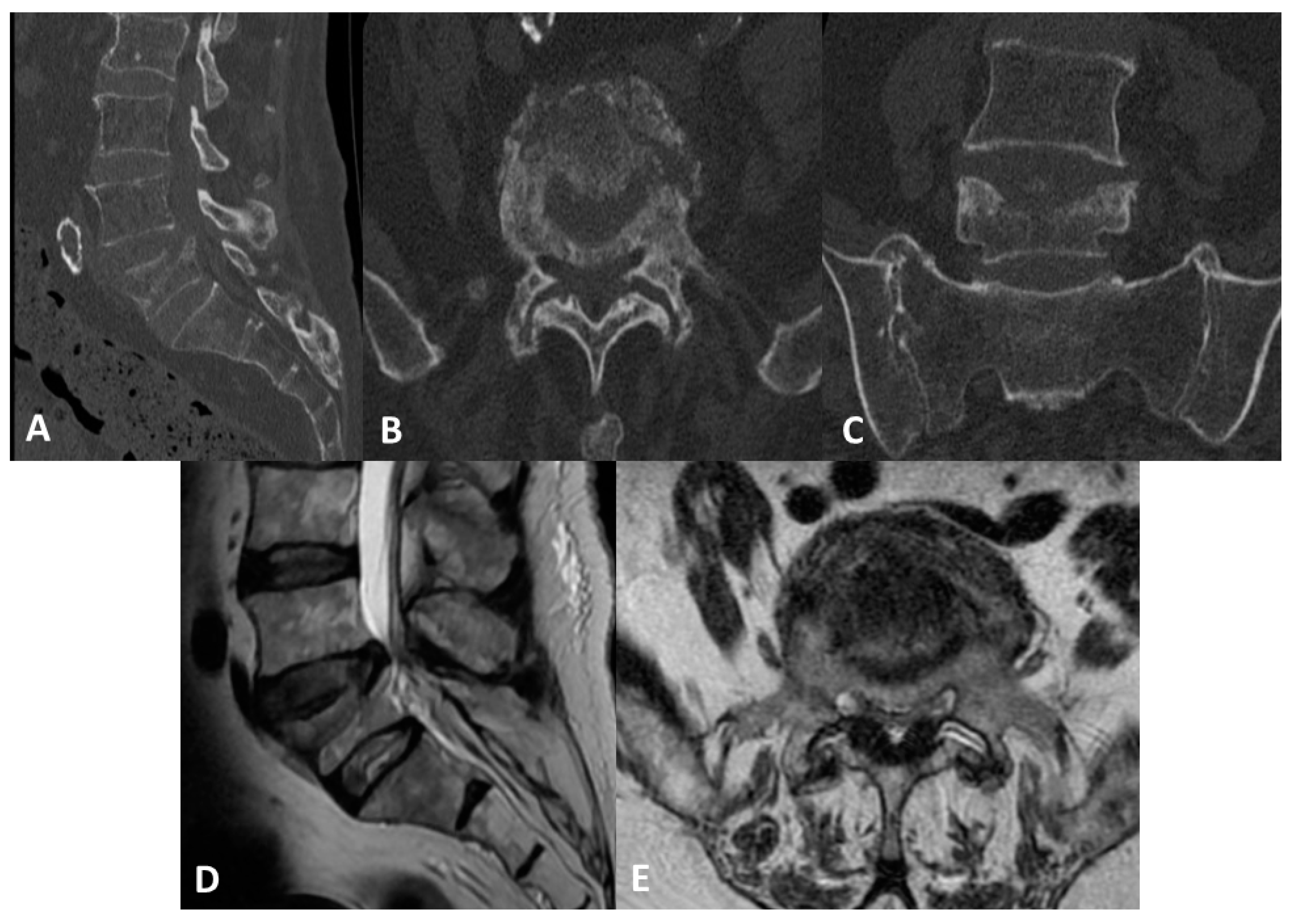
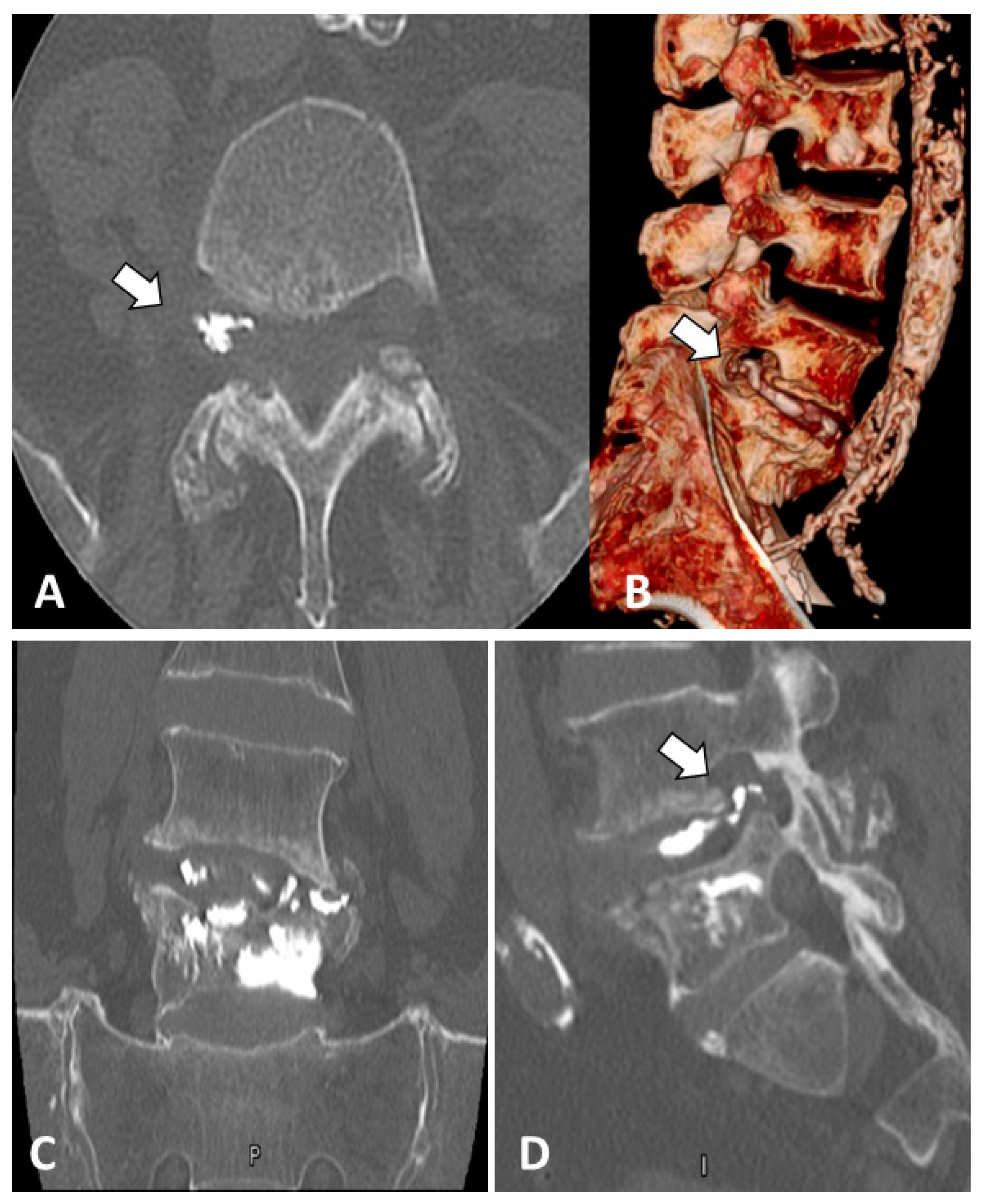
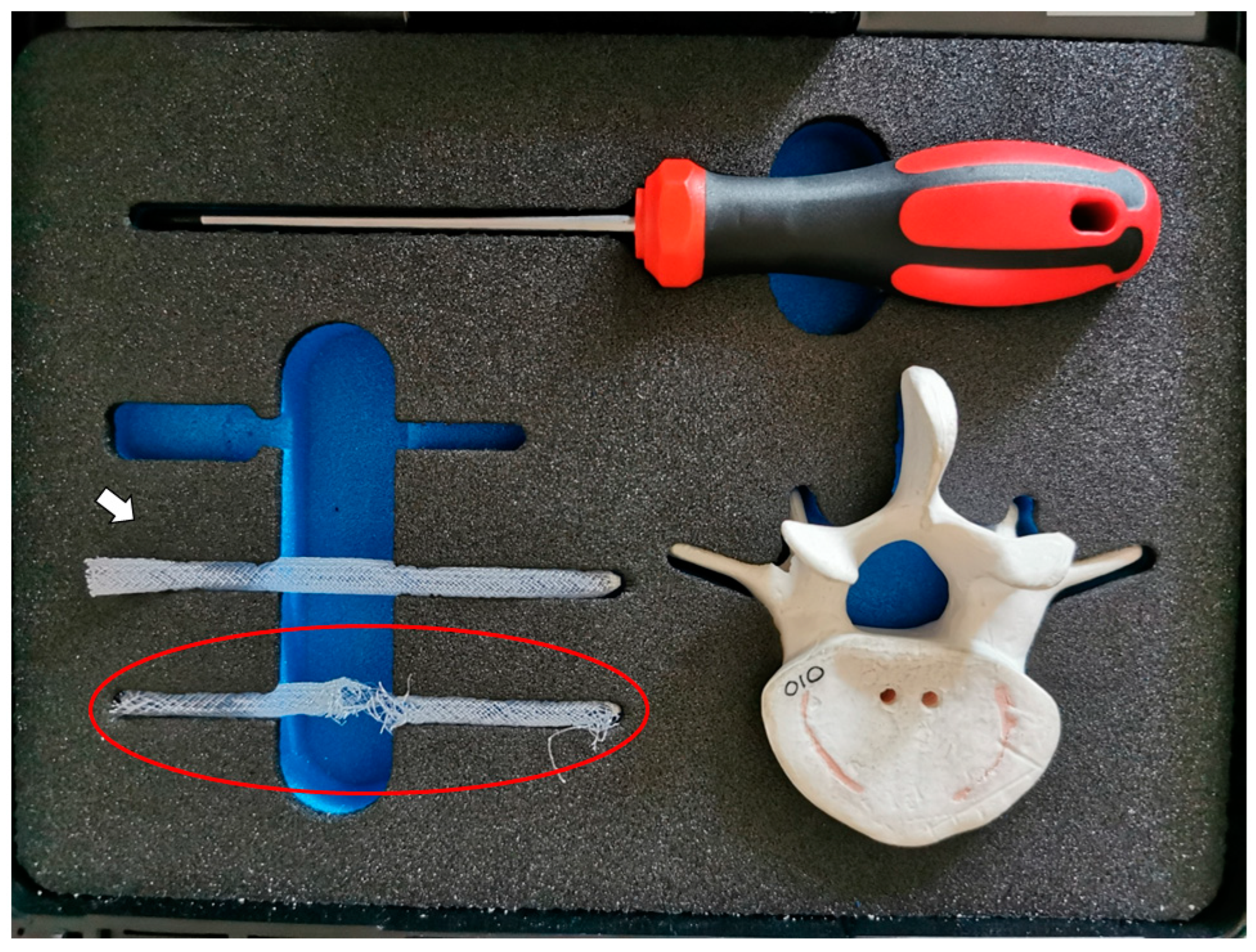
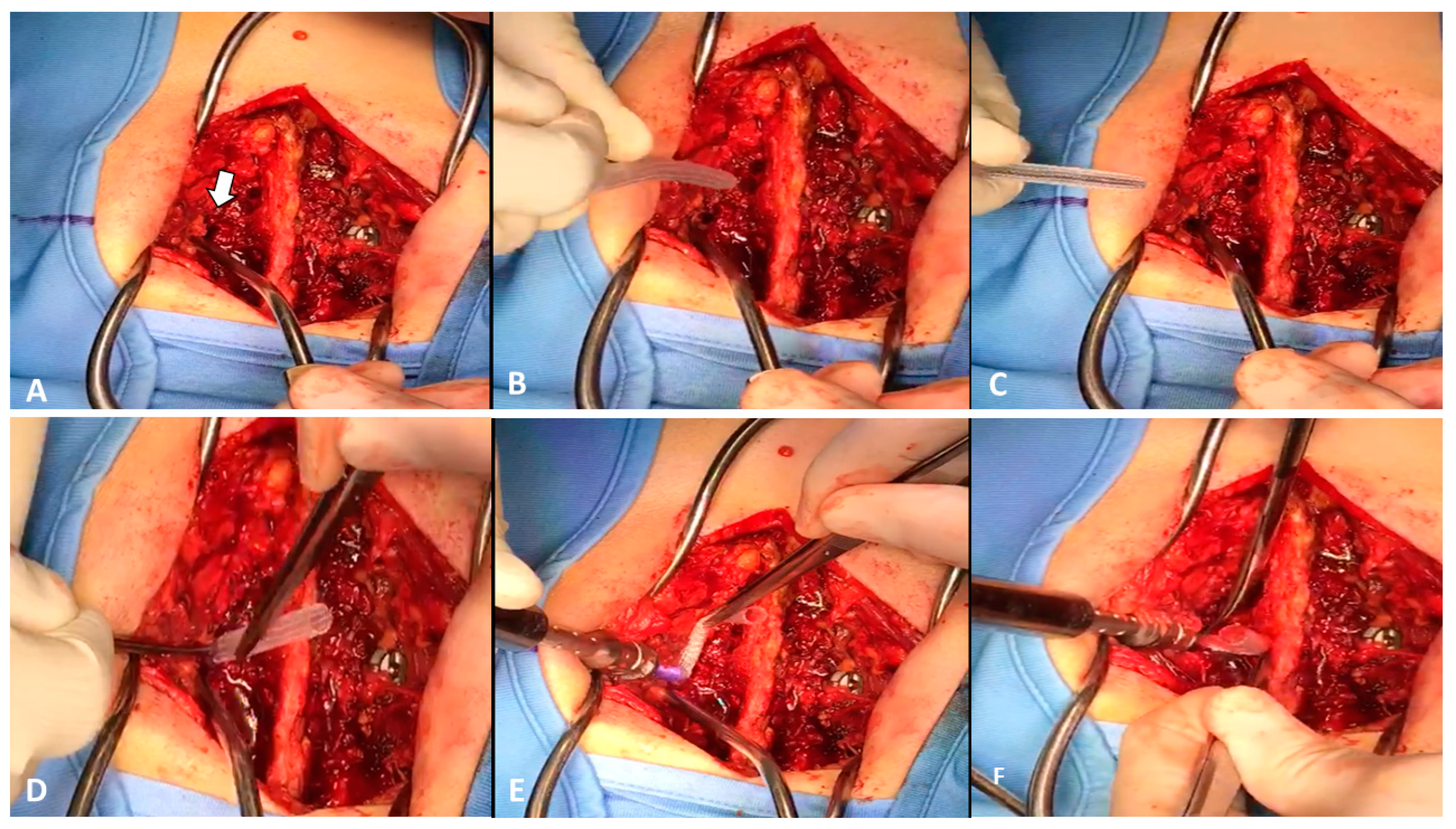
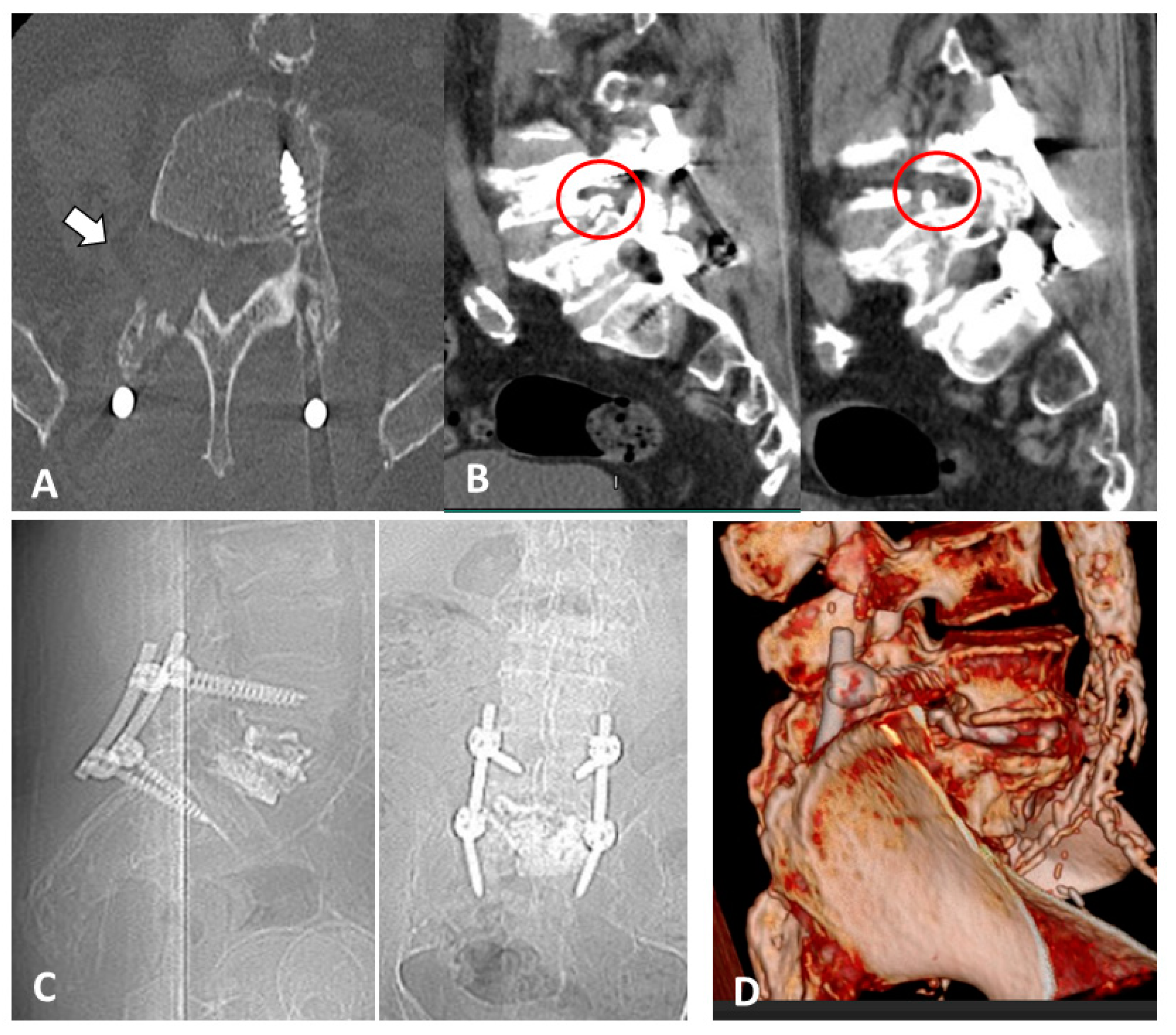
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elder, B.D.; Lo, S.-F.L.; Holmes, C.; Goodwin, C.R.; Kosztowski, T.A.; Lina, I.A.; Locke, J.E.; Witham, T.F. The biomechanics of pedicle screw augmentation with cement. Spine J. 2015, 15, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- de Kater, E.P.; Sakes, A.; Edström, E.; Elmi-Terander, A.; Kraan, G.; Breedveld, P. Beyond the pedicle screw—A patent review. Eur. Spine J. 2022, 31, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Zuckerman, S.L.; Cerpa, M.; Yeom, J.S.; Lehman, J.R.A.; Lenke, L.G. Incidence and Risk Factors for Complications and Mortality After Vertebroplasty or Kyphoplasty in the Osteoporotic Vertebral Compression Fracture—Analysis of 1,932 Cases from the American College of Surgeons National Surgical Quality Improvement. Glob. Spine J. 2020, 12, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Ahern, D.P.; McDonnell, J.M.; Riffault, M.; Evans, S.; Wagner, S.C.; Vaccaro, A.R.; Hoey, D.A.; Butler, J.S. A meta-analysis of the diagnostic accuracy of Hounsfield units on computed topography relative to dual-energy X-ray absorptiometry for the diagnosis of osteoporosis in the spine surgery population. Spine J. 2021, 21, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Semaan, H.; Obri, T.; Bazerbashi, M.; Paull, D.; Liu, X.; Sarrouj, M.; Elgafy, H. Clinical outcome and subsequent sequelae of cement extravasation after percutaneous kyphoplasty and vertebroplasty: A comparative review. Acta Radiol. 2017, 59, 861–868. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Fritzell, P. Balloon Kyphoplasty and Vertebroplasty for Vertebral Compression Fractures A Comparative Systematic Review of Efficacy and Safety. Spine 2016, 31, 2747–2755. [Google Scholar] [CrossRef]
- A Hulme, P.; Krebs, J.; Ferguson, S.J.; Berlemann, U. Vertebroplasty and Kyphoplasty: A Systematic Review of 69 Clinical Studies. Spine 2006, 31, 1983–2001. [Google Scholar] [CrossRef]
- Bohl, M.A.; Sethi, R.; Leveque, J.-C. Incidence and Clinical Risk of Cement Extravasation in Adult Patients Undergoing Prophylactic Vertebroplasty During Surgical Spine Reconstruction. World Neurosurg. 2019, 134, e928–e936. [Google Scholar] [CrossRef]
- Lador, R.; Dreiangel, N.; Ben-Galim, P.J.; Hipp, J.A. A pictorial classification atlas of cement extravasation with vertebral augmentation. Spine J. 2010, 10, 1118–1127. [Google Scholar] [CrossRef]
- Jing, Z.; Li, L.; Song, J. Delayed neurological deficits caused by cement extravasation following vertebroplasty: A case report. J. Int. Med. Res. 2021, 49, 3000605211019664. [Google Scholar] [CrossRef]
- Tuan, T.A.; Van Luong, T.; Cuong, P.M.; Long, V.; Huy, H.Q.; Duc, N.M. Cement Leakage in Percutaneous Vertebroplasty for Multiple Osteoporotic Vertebral Compression Fractures: A Prospective Cohort Study. Orthop. Res. Rev. 2020, 12, 105–111. [Google Scholar] [CrossRef]
- Patel, N.; Jacobs, D.; John, J.; Fayed, M.; Nerusu, L.; Tandron, M.; Dailey, W.; Ayala, R.; Sibai, N.; Forrest, P.; et al. Balloon Kyphoplasty vs Vertebroplasty: A Systematic Review of Height Restoration in Osteoporotic Vertebral Compression Fractures. J. Pain Res. 2022, 15, 1233–1245. [Google Scholar] [CrossRef]
- Yang, S.-C.; Chen, W.-J.; Yu, S.-W.; Tu, Y.-K.; Kao, Y.-H.; Chung, K.-C. Revision strategies for complications and failure of vertebroplasties. Eur. Spine J. 2008, 17, 982–988. [Google Scholar] [CrossRef]
- Wagner, R.; Telfeian, A.E.; Iprenburg, M.; Krzok, G.; Gokaslan, Z.; Choi, D.B.; Pucci, F.G.; Oyelese, A. Transforaminal Endoscopic Solution to a Kyphoplasty Complication: Technical Note. World Neurosurg. 2016, 91, 195–198. [Google Scholar] [CrossRef]
- Şentürk, S.; Ünsal, Ü.Ü. Percutaneous endoscopic translaminar approach in a patient with pedicle screw malposition and cement leakage. Br. J. Neurosurg. 2021. [Google Scholar] [CrossRef]
- Philips, G.A.C.; Oshima, Y.; Inoue, H.; Kitagawa, T.; Iwai, H.; Takano, Y.; Inanami, H.; Koga, H. Full-endoscopic spine surgery for radiculopathy after osteoporotic vertebral compression fractures: A case report. J. Spine Surg. 2020, 6, 466–471. [Google Scholar] [CrossRef]
- Mesfin, A.; Komanski, C.B.; Khanna, A.J. Failure of Cement-Augmented Pedicle Screws in the Osteoporotic Spine. Geriatr. Orthop. Surg. Rehabil. 2013, 4, 84–88. [Google Scholar] [CrossRef]
- Ulivieri, F.M.; Rinaudo, L. The Bone Strain Index: An Innovative Dual X-ray Absorptiometry Bone Strength Index and Its Helpfulness in Clinical Medicine. J. Clin. Med. 2022, 11, 2284. [Google Scholar] [CrossRef]
- Hauser, B.; Alonso, N.; Riches, P. Review of Current Real-World Experience with Teriparatide as Treatment of Osteoporosis in Different Patient Groups. J. Clin. Med. 2021, 10, 1403. [Google Scholar] [CrossRef]
- Ayub, N.; Faraj, M.; Ghatan, S.; Reijers, J.A.A.; Napoli, N.; Oei, L. The Treatment Gap in Osteoporosis. J. Clin. Med. 2021, 10, 3002. [Google Scholar] [CrossRef]
- Easley, J.; Puttlitz, C.M.; Seim, H.; Ramo, N.; Abjornson, C.; Cammisa, F.P.; McGilvray, K.C. Biomechanical and histologic assessment of a novel screw retention technology in an ovine lumbar fusion model. Spine J. 2018, 18, 2302–2315. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drago, G.; Pastorello, G.; Gallinaro, P.; Zanata, R.; Del Verme, J.; Stafa, A.; Giordan, E. Novel Polyethylene Terephthalate Screw Sleeve Implant: Salvage Treatment in a Case of Spine Instability after Vertebroplasty Failure. Medicines 2023, 10, 6. https://doi.org/10.3390/medicines10010006
Drago G, Pastorello G, Gallinaro P, Zanata R, Del Verme J, Stafa A, Giordan E. Novel Polyethylene Terephthalate Screw Sleeve Implant: Salvage Treatment in a Case of Spine Instability after Vertebroplasty Failure. Medicines. 2023; 10(1):6. https://doi.org/10.3390/medicines10010006
Chicago/Turabian StyleDrago, Giacomo, Giulia Pastorello, Paolo Gallinaro, Roberto Zanata, Jacopo Del Verme, Altin Stafa, and Enrico Giordan. 2023. "Novel Polyethylene Terephthalate Screw Sleeve Implant: Salvage Treatment in a Case of Spine Instability after Vertebroplasty Failure" Medicines 10, no. 1: 6. https://doi.org/10.3390/medicines10010006
APA StyleDrago, G., Pastorello, G., Gallinaro, P., Zanata, R., Del Verme, J., Stafa, A., & Giordan, E. (2023). Novel Polyethylene Terephthalate Screw Sleeve Implant: Salvage Treatment in a Case of Spine Instability after Vertebroplasty Failure. Medicines, 10(1), 6. https://doi.org/10.3390/medicines10010006






