Efficacy and Safety of Renal Function on Edoxaban Versus Warfarin for Atrial Fibrillation: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Study Search and Research Evaluation
3.2. Risk of Bias in Included Articles
3.3. Pooled Effect Estimates
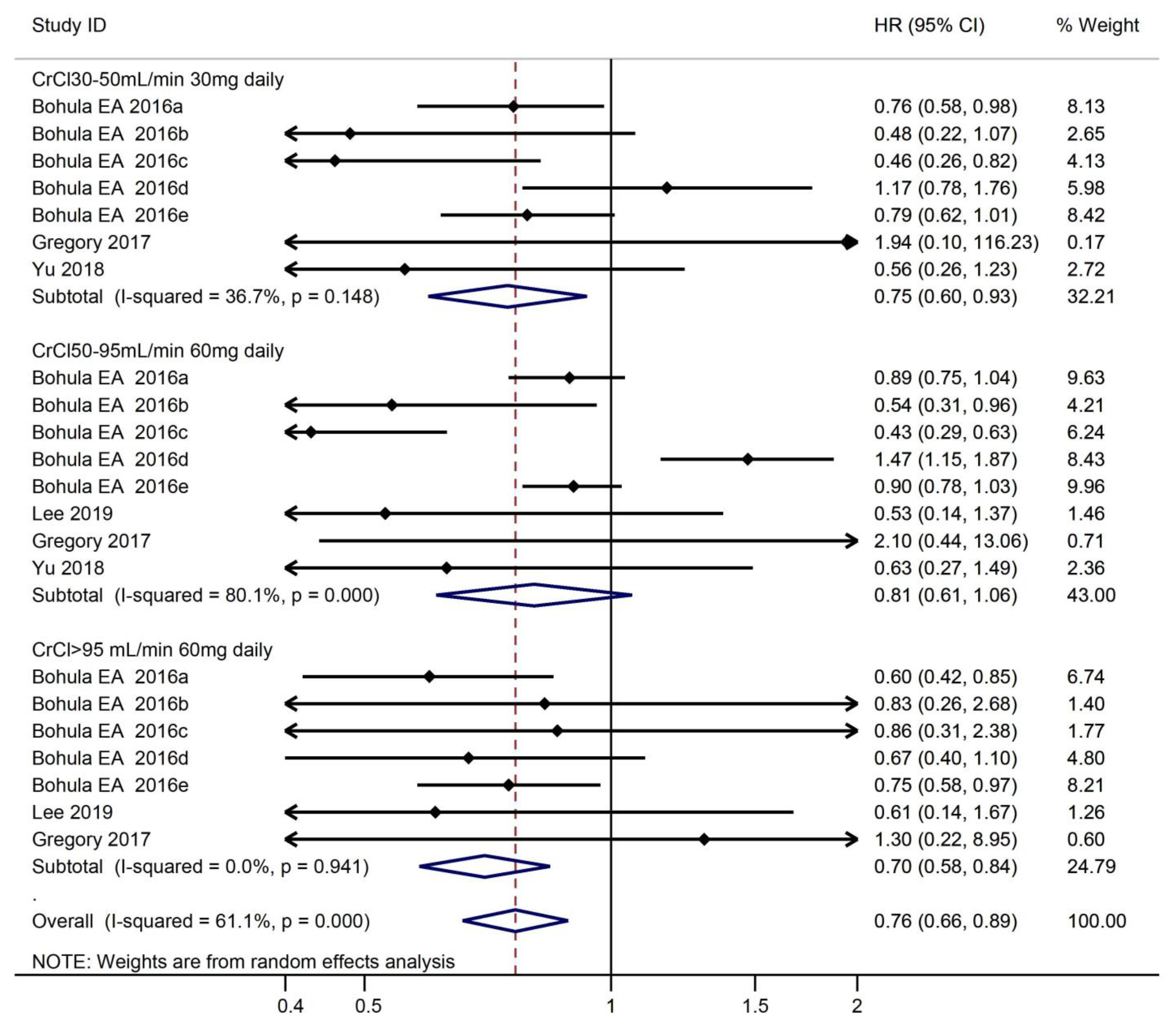
3.3.1. Safety Outcomes According to CrCl
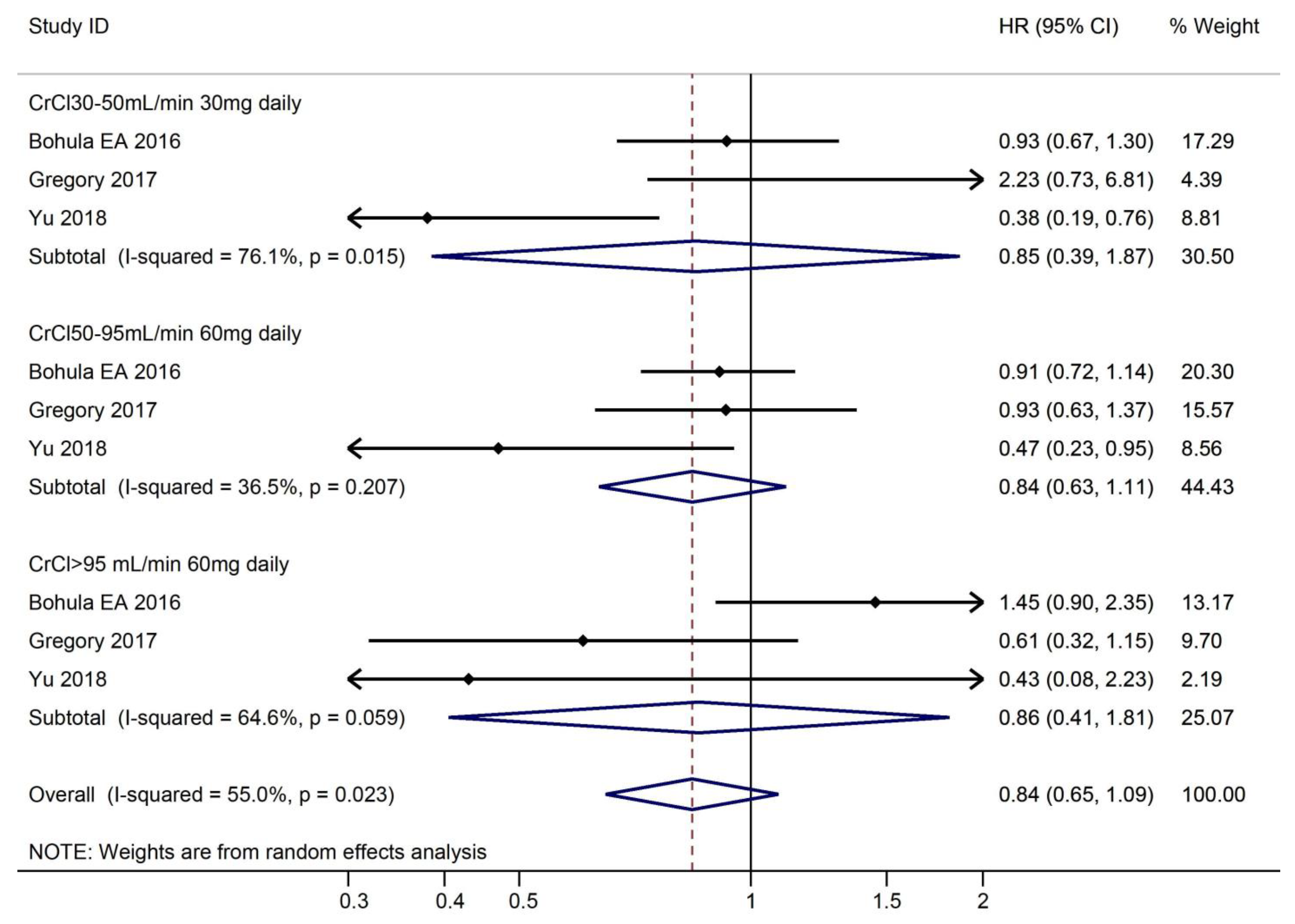
3.3.2. Efficacy Outcomes According to CrCl
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, B.V.; Brugada, B.J.; Camm, C.J.; et al. 2017 hrs/ehra/ecas/aphrs/solaece expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Giugliano, R.P.; Claggett, B.; Gupta, D.K.; Chandra, A.; Ruff, C.T.; Antman, E.M.; Mercuri, M.F.; Grosso, M.A.; Braunwald, B.E.; et al. Left atrial structure and function and the risk of death or heart failure in atrial fibrillation. Eur. J. Heart Fail. 2019, 21, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Li, Z.X.; Gu, H.Q.; Zhai, Y.; Jiang, Y.; Zhao, X.Q.; Wang, Y.L.; Yang, X.; Wang, C.J.; Meng, X.; et al. China stroke statistics 2019: A report from the national center for healthcare quality management in neurological diseases, china national clinical research center for neurological diseases, the chinese stroke association, national center for chronic and non-communicable disease control and prevention, chinese center for disease control and prevention and institute for global neuroscience and stroke collaborations. Stroke Vasc. Neurol. 2020, 5, 211–239. [Google Scholar] [PubMed]
- Murray-Bruce, D. Age and ageing: An overview. Occup. Med. (Oxf. Engl. ) 2000, 50, 471–472. [Google Scholar] [CrossRef]
- Mulder, F.I.; Bosch, F.T.M.; Young, A.M.; Marshall, A.; McBane, R.D.; Zemla, T.J.; Carrier, M.; Kamphuisen, P.W.; Bossuyt, P.M.M.; Büller, H.R.; et al. Direct oral anticoagulants for cancer-associated venous thromboembolism: A systematic review and meta-analysis. Blood 2020, 136, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Vranckx, P. Atrial fibrillation patients undergoing percutaneous coronary intervention: Dual or triple antithrombotic therapy with non-vitamin k antagonist oral anticoagulants. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2020, 22, I22–I31. [Google Scholar] [CrossRef]
- Administration UFaD. Prescribing information for savaysa (edoxaban). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf.2015 (accessed on 8 January 2015).
- Bohula, E.A.; Giugliano, R.P.; Ruff, C.T.; Kuder, J.F.; Murphy, S.A.; Antman, E.M.; Braunwald, E. Impact of renal function on outcomes with edoxaban in the engage af-timi 48 trial. Circulation 2016, 134, 24–36. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. Robins-i: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Al-Saady, N.; Ezekowitz, M.D.; Banach, M.; Goette, A. The relationship of renal function to outcome: A post hoc analysis from the edoxaban versus warfarin in subjects undergoing cardioversion of atrial fibrillation (ensure-af) study. Am. Heart J. 2017, 193, 16–22. [Google Scholar] [CrossRef]
- Lee, S.R.; Choi, E.K.; Han, K.D.; Jung, J.H.; Cha, M.J.; Oh, S.; Lip, G.Y. Non-vitamin k antagonist oral anticoagulants in asian patients with supranormal renal function. Stroke 2019, 50, 1480–1489. [Google Scholar] [CrossRef]
- Yu, H.T.; Yang, P.S.; Kim, T.H.; Jang, E.; Kim, D.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; Lip, G.Y.H.; et al. Impact of renal function on outcomes with edoxaban in real-world patients with atrial fibrillation. Stroke 2018, 49, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.B.; Lane, D.A.; Rasmussen, L.H.; Lip, G.Y.; Larsen, T.B. Renal function and non-vitamin k oral anticoagulants in comparison with warfarin on safety and efficacy outcomes in atrial fibrillation patients: A systemic review and meta-regression analysis. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2015, 104, 418–429. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Diener, H.C.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; Camm, A.J.; Kirchhof, K.P.; et al. Updated european heart rhythm association practical guide on the use of non-vitamin k antagonist anticoagulants in patients with non-valvular atrial fibrillation. Ep Eur. 2015, 17, 1467–1507. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Dalen, J.; Anderson, D.R.; Poller, L.; Bussey, H.; Ansell, J.; Deykin, D. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001, 119, 8s–21s. [Google Scholar] [CrossRef]
- Raval, A.N.; Cigarroa, J.E.; Chung, M.K.; Diaz-Sandoval, L.J.; Diercks, D.; Piccini, J.P.; Jung, H.S.; Washam, J.B.; Welch, B.G.; Zazulia, A.R.; et al. Management of patients on non-vitamin k antagonist oral anticoagulants in the acute care and periprocedural setting: A scientific statement from the american heart association. Circulation 2017, 135, e604–e633. [Google Scholar] [CrossRef]
- Banerjee, A.; Fauchier, L.; Vourc’h, P.; Andres, C.R.; Taillandier, S.; Halimi, J.M.; Lip, G.Y. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: The loire valley atrial fibrillation project. J. Am. Coll. Cardiol. 2013, 61, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Ishfaq, M.F.; Goyal, N.; Katsanos, A.H.; Parissis, J.; Alexandrov, A.W.; Alexandrov, A.V.; Tsivgoulis, T.G. Oral anticoagulation in patients with chronic kidney disease: A systematic review and meta-analysis. Neurology 2019, 92, e2421–e2431. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, R.H.; Roldan-Schilling, V.; et al. The 2018 european heart rhythm association practical guide on the use of non-vitamin k antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, M.; Ge, S.; Feng, J. Mechanical valve replacement without anticoagulation: A case report. Eur. Heart J. Case Rep. 2021, 5, ytaa566. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, P.; Valgimigli, M.; Eckardt, L.; Tijssen, J.; Lewalter, T.; Gargiulo, G.; Batushkin, V.; Campo, G.; Lysak, Z.; Vakaliuk, I.; et al. Edoxaban-based versus vitamin k antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (entrust-af pci): A randomised, open-label, phase 3b trial. Lancet 2019, 394, 1335–1343. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; Meyer, G.; et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Zou, R.; Tao, J.; Shi, W.; Yang, M.; Li, H.; Lin, X.; Yang, S.; Hua, P. Meta-analysis of safety and efficacy for direct oral anticoagulation treatment of non-valvular atrial fibrillation in relation to renal function. Thromb. Res. 2017, 160, 41–50. [Google Scholar] [CrossRef]






| Characteristics | Trial | |||
|---|---|---|---|---|
| 2016 Bohula, E.A. | 2017 Lip, Gregory | 2018 Hee Tae | 2019 So-Ryoung Lee | |
| Country | International | International | Korea | Korea |
| Design | Multinational, randomized, double-blind | RCT | Retrospectively | Retrospective nationwide cohort study |
| Registry | NCT00781391 | NCT02072434 | NA | NCT02786095 |
| Number of Patients | 14,071 | 1095 | 11,712 | 11,071 |
| Endpoints/second outcome | Endpoints: stroke or systemic embolism major bleeding all-cause death; Additional safety end points: intracranial hemorrhage, gastrointestinal bleeding, minor bleeding. | Endpoints: stroke, systemic embolic event, myocardial infarction, any bleeding, cardiovascular death. | Endpoints: stroke or systemic embolism, major bleeding, and death from any cause; Secondary outcomes: intracranial bleeding, gastrointestinal bleeding, myocardial infarction, or admission for heart failure. | Endpoints: ischemic stroke, major bleeding, all-cause death. |
| CrCl, mL/min | 30–50, 50–95, >95 | 15–30, 30–50, 50–80, 80–95, ≥95 | 30–50, 50–70, 70–95, >95, | 80–95, >95 |
| Follow Up | 2.8 (interquartile range, 2.4–3.2 years) | 28 days | 5.0 months (interquartile range, 2–7 months) | 1.2 years (interquartile range, 0.6–1.9 years) |
| CHADS2 risk score | 2.8 | 2.6 | 4.2 ± 1.7 | 3.0 ± 1.6 |
| Medical history | Edoxaban 30 mg vs. Edoxaban 60 mg vs. Warfarin Diabetes: 1:1:1 Hypertension: 1:1:1 Heart failure: 1:1:1 Ischaemic stroke or transient ischaemic attack: 1:1:1 Previous VKA used: 1:1:1 | Edoxaban vs. Warfarin Congestive heart failure:1:1 Coronary artery disease: 1:1 Hypertension: 1:1 Diabetes: 1:1 Ischaemic heart disease: 1:1 Ischaemic stroke or transient ischaemic attack: 1:1 Life-threatening bleed: 1:1 | Edoxaban 30 mg vs. edoxaban 60 mg vs. Warfarin Diabetes: 1:1:1 Hypertension: 1:1:1 Heart failure: 1:1:1 Ischaemic stroke or transient ischaemic attack: 1:1:1 Vascular disease: 1:1:1 Dyslipidemia: 1:1:1 | Edoxaban vs. Warfarin Heart failure: 1:1 Dyslipidemia: 1:1 Hypertension: 1:1 Diabetes: 1:1 Myocardial infarction: 1:1 Peripheral artery disease: 1:1 Chronic obstructive pulmonary disease: 1:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, L.; Wei, Z.; Lu, S.; Liu, W.; Zhang, J.; Feng, J.; Wang, D. Efficacy and Safety of Renal Function on Edoxaban Versus Warfarin for Atrial Fibrillation: A Systematic Review and Meta-Analysis. Medicines 2023, 10, 13. https://doi.org/10.3390/medicines10010013
Wang Y, Li L, Wei Z, Lu S, Liu W, Zhang J, Feng J, Wang D. Efficacy and Safety of Renal Function on Edoxaban Versus Warfarin for Atrial Fibrillation: A Systematic Review and Meta-Analysis. Medicines. 2023; 10(1):13. https://doi.org/10.3390/medicines10010013
Chicago/Turabian StyleWang, Yapeng, Li Li, Zhanlan Wei, Shan Lu, Wenxue Liu, Janghui Zhang, Junbo Feng, and Dongjin Wang. 2023. "Efficacy and Safety of Renal Function on Edoxaban Versus Warfarin for Atrial Fibrillation: A Systematic Review and Meta-Analysis" Medicines 10, no. 1: 13. https://doi.org/10.3390/medicines10010013
APA StyleWang, Y., Li, L., Wei, Z., Lu, S., Liu, W., Zhang, J., Feng, J., & Wang, D. (2023). Efficacy and Safety of Renal Function on Edoxaban Versus Warfarin for Atrial Fibrillation: A Systematic Review and Meta-Analysis. Medicines, 10(1), 13. https://doi.org/10.3390/medicines10010013





