Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications
Abstract
1. Introduction
Literature Searching Strategy
2. Discussion
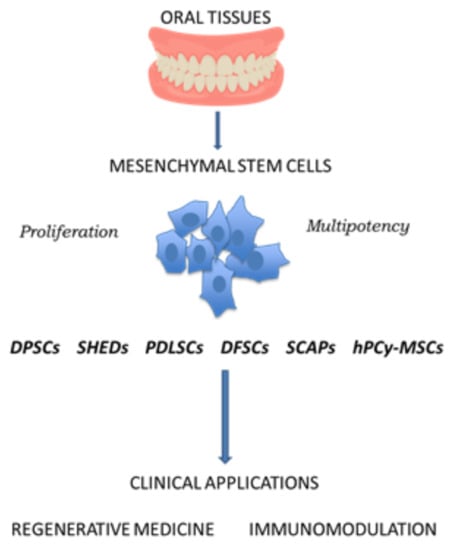
2.1. Oral-Derived Mesenchymal Stem Cells
2.2. The Human Periapical Cyst-Mesenchymal Stem Cells (hPCy-MSCs)
2.3. Tissue Engineering and Regenerative Medicine (TERM)
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DPSCs | Pulp Stem Cells |
| SHEDs | Human Deciduous Teeth Stem Cells |
| PDLSCs | Periodontal Ligament Stem Cells |
| DFSCs | Dental Follicle Stem Cells |
| SCAPs | Stem Cells from Apical Papilla |
| hPCy-MSCs | human Periapical Cysts-Mesenchymal Stem Cells |
References
- Munoz-Canoves, P.; Huch, M. Definitions for adult stem cells debated. Nature 2018, 563, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Paduano, F. The regenerative medicine in oral and maxillofacial surgery: The most important innovations in the clinical application of mesenchymal stem cells. Int. J. Med. Sci. 2015, 12, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Becchetti, E.; Marchionni, C.; Alviano, F.; Fossati, V.; et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Yamaza, T.; Kentaro, A.; Chen, C.; Liu, Y.; Shi, Y.; Gronthos, S.; Wang, S.; Shi, S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res. Ther. 2010, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Zheng, L.; Wake, H.; Ito, M.; Nabekura, J.; Wakita, H.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. A novel stem cell source for vasculogenesis in ischemia: Subfraction of side population cells from dental pulp. Stem Cells 2008, 26, 2408–2418. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Paduano, F.; Tatullo, M. Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. J. Dent. Res. 2015, 94, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Falisi, G.; Amantea, M.; Rastelli, C.; Paduano, F.; Marrelli, M. Dental pulp stem cells and human periapical cyst mesenchymal stem cells in bone tissue regeneration: Comparison of basal and osteogenic differentiated gene expression of a newly discovered mesenchymal stem cell lineage. J. Biol. Regul. Homeost. Agents 2015, 29, 713–718. [Google Scholar] [PubMed]
- Till, J.E.; Mc, C.E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961, 14, 213–222. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part II: Clinical applications. J. Prosthodont. Res. 2012, 56, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Isakova, I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr. Pharm. Des. 2005, 11, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Catacchio, I.; Berardi, S.; Reale, A.; De Luisi, A.; Racanelli, V.; Vacca, A.; Ria, R. Evidence for bone marrow adult stem cell plasticity: Properties, molecular mechanisms, negative aspects, and clinical applications of hematopoietic and mesenchymal stem cells transdifferentiation. Stem Cells Int. 2013, 2013, 589139. [Google Scholar] [CrossRef] [PubMed]
- Beyer Nardi, N.; da Silva Meirelles, L. Mesenchymal stem cells: Isolation, in vitro expansion and characterization. Handb. Exp. Pharmacol. 2006, 249–282. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (dpscs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Ishkitiev, N.; Yaegaki, K.; Calenic, B.; Nakahara, T.; Ishikawa, H.; Mitiev, V.; Haapasalo, M. Deciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitro. J. Endod. 2010, 36, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, M.; Lee, C.H.; Yoon, R.; Lal, S.; Mao, J.J. Clones of ectopic stem cells in the regeneration of muscle defects in vivo. PLoS ONE 2010, 5, e13547. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Zheng, L.; Ito, M.; Tomokiyo, A.; Matsushita, K.; Nakashima, M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells 2006, 24, 2493–2503. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Jahoda, C.A. Cultured human and rat tooth papilla cells induce hair follicle regeneration and fiber growth. Differentiation 2004, 72, 566–575. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. Shed: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Morsczeck, C.; Gotz, W.; Schierholz, J.; Zeilhofer, F.; Kuhn, U.; Mohl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (pcs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Qiu, Y.; Zhou, N.; Ouyang, H.; Ding, J.; Cheng, B.; Sun, J. Application of stem cells in oral disease therapy: Progresses and perspectives. Front. Physiol. 2017, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Sittinger, M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003, 9 (Suppl. 1), S45–S64. [Google Scholar] [CrossRef]
- Yi, T.; Lee, S.; Choi, N.; Shin, H.S.; Kim, J.; Lim, J.Y. Single cell clones purified from human parotid glands display features of multipotent epitheliomesenchymal stem cells. Sci. Rep. 2016, 6, 36303. [Google Scholar] [CrossRef]
- Marrelli, M.; Paduano, F.; Tatullo, M. Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. Int. J. Biol. Sci. 2013, 9, 1070–1078. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Palmieri, F.; Tatullo, M. Cd146 expression influences periapical cyst mesenchymal stem cell properties. Stem Cell Rev. 2016, 12, 592–603. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Pacifici, A.; Palmieri, F.; Marrelli, M.; Pacifici, L.; Paduano, F. Potential use of human periapical cyst-mesenchymal stem cells (hpcy-mscs) as a novel stem cell source for regenerative medicine applications. Front. Cell Dev. Biol. 2017, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2003, 5, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Cyranoski, D. ‘Reprogrammed’ stem cells to be tested in people with Parkinson’s. Nature 2018. [Google Scholar] [CrossRef]
- Codispoti, B.; Marrelli, M.; Paduano, F.; Tatullo, M. Nanometric bio-banked msc-derived exosome (nanobiome) as a novel approach to regenerative medicine. J. Clin. Med. 2018, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental Pulp Stem Cell Mechanoresponsiveness: Effects of Mechanical Stimuli on Dental Pulp Stem Cell Behavior. Front. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnuolo, G.; Codispoti, B.; Marrelli, M.; Rengo, C.; Rengo, S.; Tatullo, M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dent. J. 2018, 6, 72. https://doi.org/10.3390/dj6040072
Spagnuolo G, Codispoti B, Marrelli M, Rengo C, Rengo S, Tatullo M. Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dentistry Journal. 2018; 6(4):72. https://doi.org/10.3390/dj6040072
Chicago/Turabian StyleSpagnuolo, Gianrico, Bruna Codispoti, Massimo Marrelli, Carlo Rengo, Sandro Rengo, and Marco Tatullo. 2018. "Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications" Dentistry Journal 6, no. 4: 72. https://doi.org/10.3390/dj6040072
APA StyleSpagnuolo, G., Codispoti, B., Marrelli, M., Rengo, C., Rengo, S., & Tatullo, M. (2018). Commitment of Oral-Derived Stem Cells in Dental and Maxillofacial Applications. Dentistry Journal, 6(4), 72. https://doi.org/10.3390/dj6040072