Clinical and Radiological Outcome of Titanium Implants in Clinical Practice: A 5 Year, Prospective, Multicenter Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Conduct
2.2. Materials and Methods
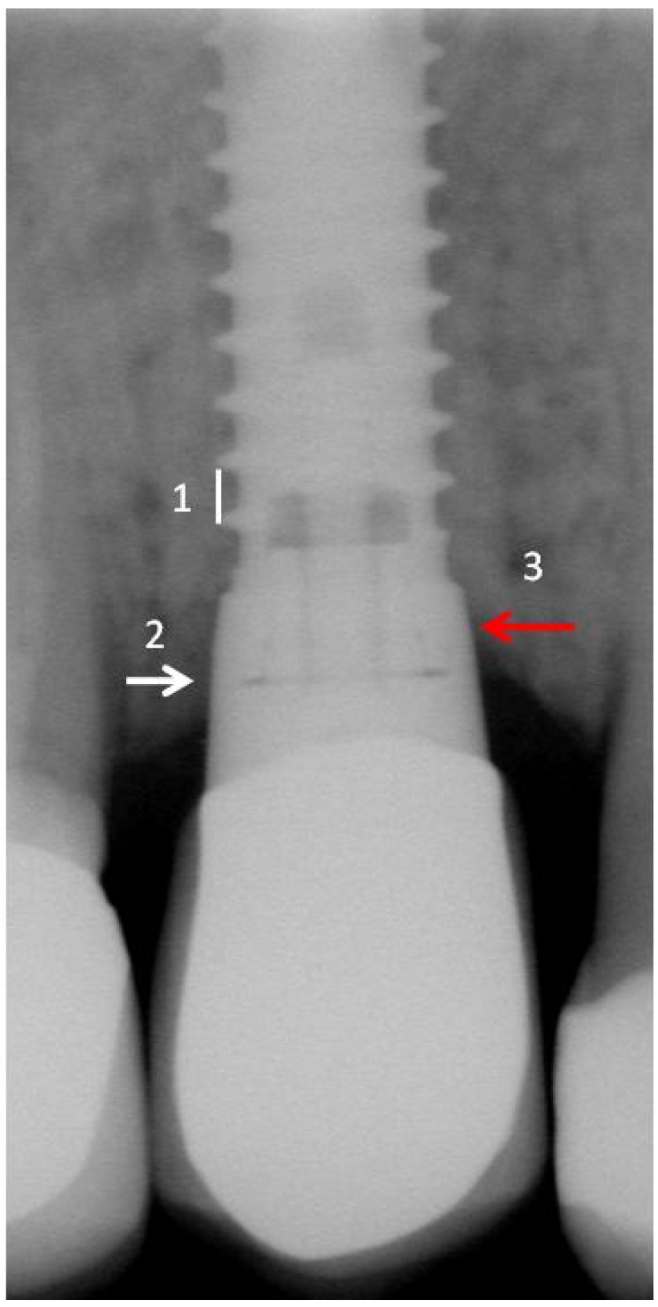
3. Results
| Risk factor | No. of patients |
|---|---|
| Smokers (patient self-assessment) | 5 of 71 (7%) |
| Previous periodontitis treatment | 12 of 71 (17%) |
| Insertion | Submerged | Non-submerged | Not known | |
| No. of sites (%)* | 86 (75) | 12 (11) | 16 (14) | |
| Simultaneous bone augmentation | Yes | No | Not known | |
| No. of sites (%)* | 67 (59) | 46 (40) | 1 (1) | |
| Bone quality | I | II | III | IV |
| No. of sites (%)*√ | 2 (2) | 69 (61) | 41 (36) | 0 (0) |
| Terminal insertion torque (Ncm) | <20 | 20–30 | 31–40 | >40 |
| No. of sites (%)*∆ | 16 (14) | 53 (46) | 11 (10) | 19 (17) |
| Restoration | No. of implants (100% = 114) |
|---|---|
| Single tooth replacement | 43 (38%) |
| Fixed bridge | 47 (42%) |
| Removable restoration | 15 (13%) |
| Not known | 9 (7%) |
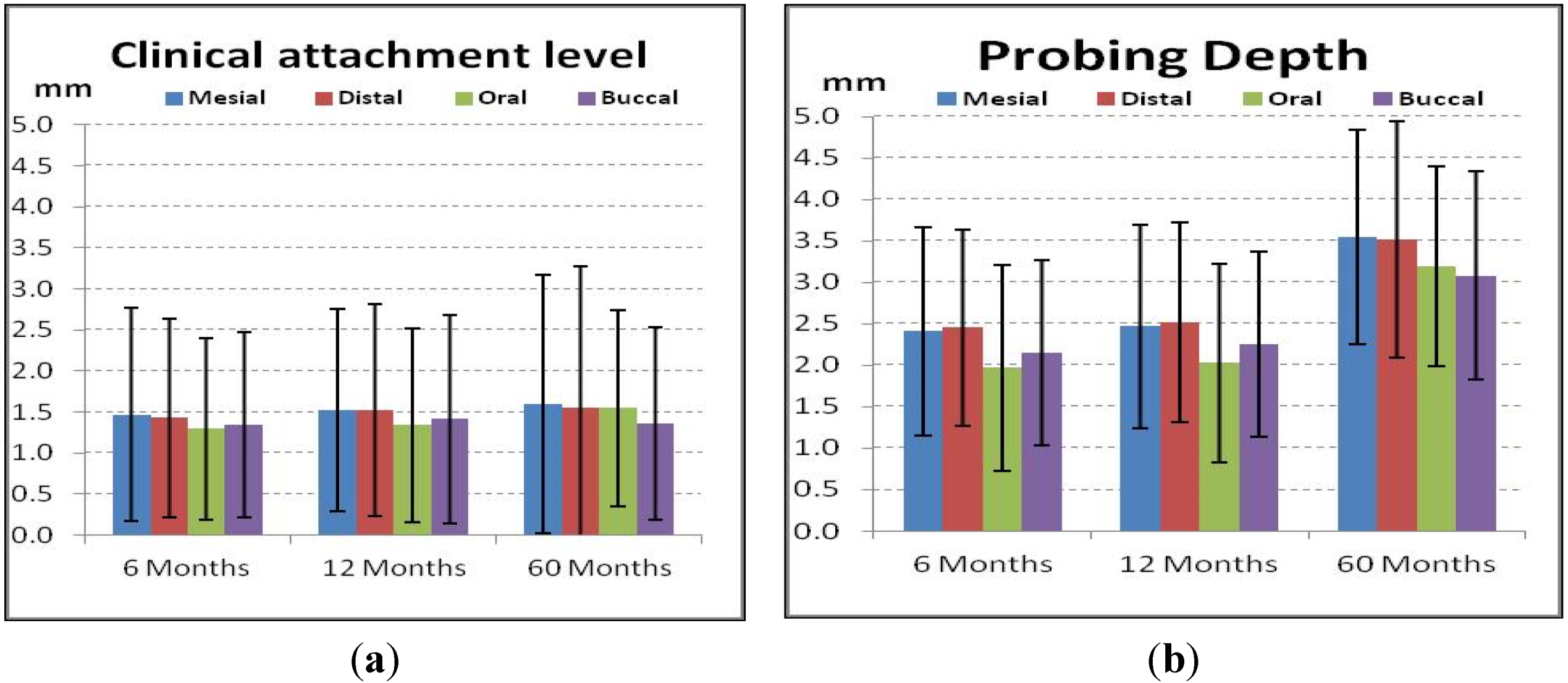
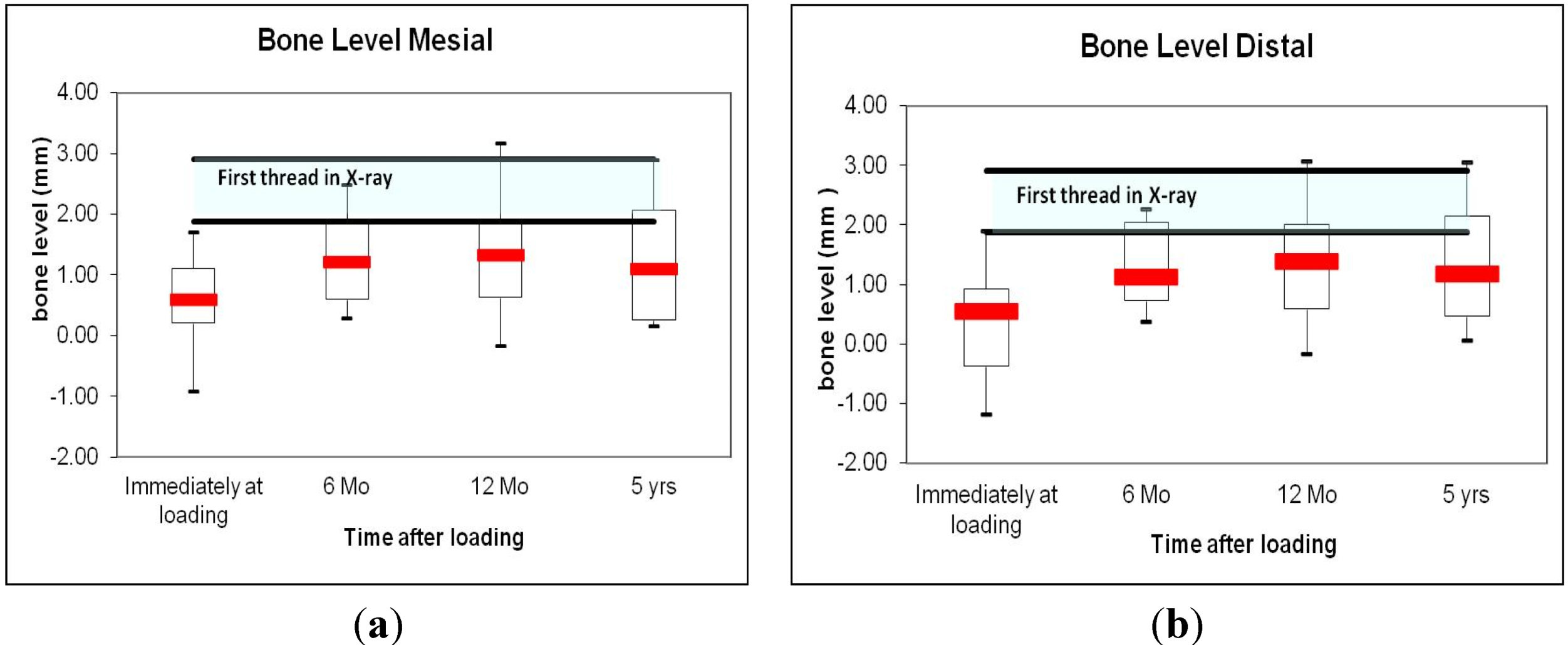
4. Discussion
4.1. Risk Factors
4.2. Surgical Characteristics

4.3. Prosthetic Parameters
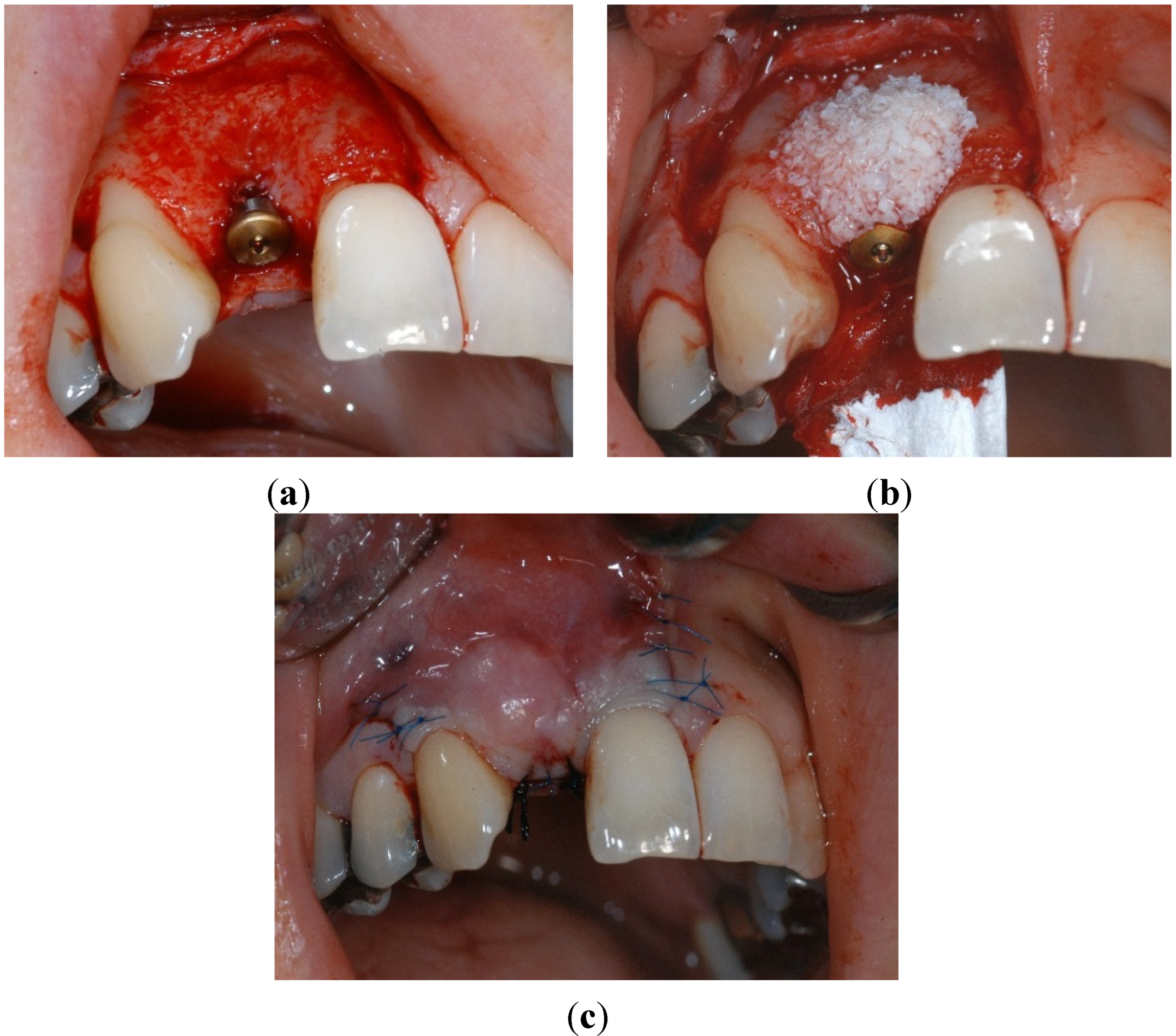
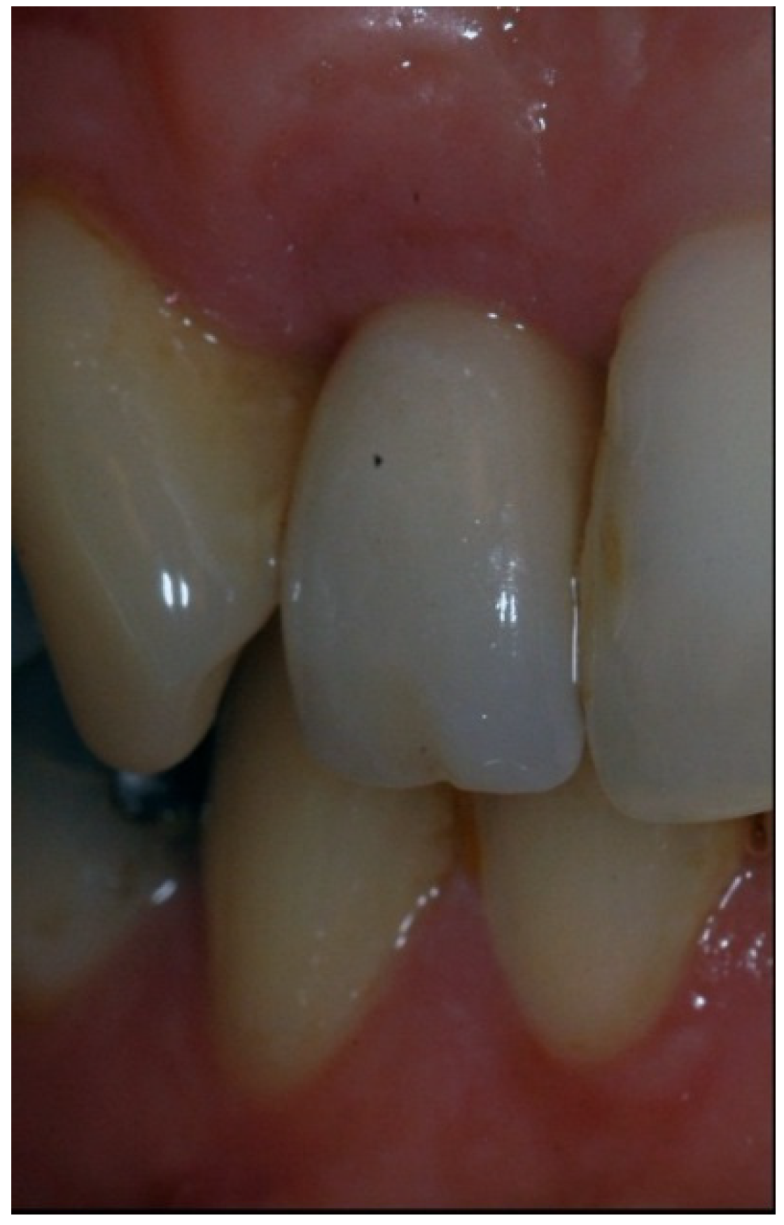
4.4. Periodontal and Radiologic Parameters
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buser, D.; Mericske-Stern, R.; Bernard, J.P.; Behneke, A.; Behneke, N.; Hirt, H.P.; Belser, U.C.; Lang, N.P. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin. Oral Implants Res. 1997, 8, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Nydegger, T.; Oxland, T.; Cochran, D.L.; Schenk, R.K.; Hirt, H.P.; Snétivy, D.; Nolte, L.-P. Interface shear strength of titanium implants with a sandblasted and acid-etched surface: A biomechanical study in the maxilla of miniature pigs. J. Biomed. Mater. Res. 1999, 45, 75–83. [Google Scholar]
- Ferguson, S.J.; Broggini, N.; Wieland, M.; de Wild, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Buser, D. Biomechanical evaluation of the interfacial strength of a chemically modified sandblasted and acid-etched titanium surface. J. Biomed. Mater. Res. A. 2006, 78, 291–297. [Google Scholar] [CrossRef]
- Langhoff, J.D.; Voelter, K.; Scharnweber, D.; Schnabelrauch, M.; Schlottig, F.; Hefti, T.; Kalchofner, K.; Nuss, K.; von Rechenberg, B. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: A study in sheep. Int. J. Oral Maxillofac. Surg. 2008, 37, 1125–1132. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A. Primary stability determination by means of insertion torque and RFA in a sample of 4,135 implants. Clin. Implant Dent. Relat. Res. 2012, 17, 501–507. [Google Scholar] [CrossRef]
- Engelberger, T.; Hefti, A.; Kallenberger, A.; Rateitschak, K.H. Correlations among Papilla Bleeding Index, other clinical indices and histologically determined inflammation of gingival papilla. J. Clin. Periodontol. 1983, 10, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Whaites, E.; Brown, J. An update on dental imaging. Br. Dent. J. 1998, 185, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B.; Flemmig, T.F. Tissue augmentation and esthetics (Working Group 3). Clin. Oral Implants Res. 2009, 20 (Suppl 4), 166–170. [Google Scholar] [CrossRef]
- Paolantoni, G.; Marenzi, G.; Fusco, A.; Sammartino, G. Implant rehabilitation of central incisor: A staged approach. Implant Dent. 2007, 16, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.K.; Hill, M. Cigarette smoking and the periodontal patient. J. Periodontol. 2004, 75, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Schwartz-Arad, D. The effect of cigarette smoking on dental implants and related surgery. Implant Dent. 2005, 14, 357–361. [Google Scholar] [PubMed]
- Mengel, R.; Behle, M.; Flores-de-Jacoby, L. Osseointegrated implants in subjects treated for generalized aggressive periodontitis: 10-year results of a prospective, long-term cohort study. J. Periodontol. 2007, 78, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Karoussis, I.K.; Salvi, G.E.; Heitz-Mayfield, L.J.; Brägger, U.; Hämmerle, C.H.; Lang, N.P. Long-term implant prognosis in patients with and without a history of chronic periodontitis: A 10-year prospective cohort study of the ITI Dental Implant System. Clin. Oral Implants Res. 2003, 14, 329–339. [Google Scholar] [CrossRef]
- Cochran, D.L.; Buser, D.; ten Bruggenkate, C.M.; Weingart, D.; Taylor, T.M.; Bernard, J.-P.; Peters, F.; Simpson, J.P. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: Early results from clinical trials on ITI SLA implants. Clin. Oral Implants Res. 2002, 13, 144–153. [Google Scholar] [CrossRef]
- Kessler-Liechti, G.; Mericske-Stern, R. Anterior teeth esthetics with the SPI implant system. Schweiz Monatsschr. Zahnmed. 2006, 116, 275–286. [Google Scholar] [PubMed]
- Buser, D.; von Arx, T. Surgical procedures in partially edentulous patients with ITI implants. Clin. Oral Implants Res. 2000, 11 (Suppl 1), 83–100. [Google Scholar] [PubMed]
- Buser, D.; Weber, H.P.; Lang, N.P. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin. Oral Implants Res. 1990, 1, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Halbritter, S.; Hart, C.; Bornstein, M.M.; Grütter, L.; Chappuis, V.; Belser, U.C. Early implant placement with simultaneous guided bone regeneration following single-tooth extraction in the esthetic zone: 12-month results of a prospective study with 20 consecutive patients. J. Periodontol. 2009, 80, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Turkyilmaz, I.; Aksoy, U.; McGlumphy, E.A. Two alternative surgical techniques for enhancing primary implant stability in the posterior maxilla: A clinical study including bone density, insertion torque, and resonance frequency analysis data. Clin. Implant Dent. Relat. Res. 2008, 10, 231–237. [Google Scholar] [PubMed]
- Ottoni, J.M.; Oliveira, Z.F.; Mansini, R.; Cabral, A.M. Correlation between placement torque and survival of single-tooth implants. Int. J. Oral Maxillofac. Implants 2005, 20, 769–776. [Google Scholar] [PubMed]
- Moheng, P.; Feryn, J.M. Clinical and biologic factors related to oral implant failure: A 2-year follow-up study. Implant Dent. 2005, 14, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Priest, G. Single-tooth implants and their role in preserving remaining teeth: A 10-year survival study. Int. J. Oral Maxillofac. Implants 1999, 14, 181–188. [Google Scholar] [PubMed]
- Hermann, J.S.; Buser, D.; Schenk, R.K.; Higginbottom, F.L.; Cochran, D.L. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin. Oral Implants Res. 2000, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Lang, N.P. Diagnostic parameters for monitoring peri-implant conditions. Int. J. Oral Maxillofac. Implants 2004, 19, 116–127. [Google Scholar] [PubMed]
- Hänggi, M.P.; Hänggi, D.C.; Schoolfield, J.D.; Meyer, J.; Cochran, D.L.; Hermann, J.S. Crestal bone changes around titanium implants. Part I: A retrospective radiographic evaluation in humans comparing two non-submerged implant designs with different machined collar lengths. J. Periodontol. 2005, 76, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Han, C.H.; Heo, S.J.; Kim, S.; Chun, H.J. Radiographic evaluation of marginal bone level around implants with different neck designs after 1 year. Int. J. Oral Maxillofac. Implants 2006, 21, 789–794. [Google Scholar] [PubMed]
- Gholami, H.; Mericske-Stern, R.; Kessler-Liechti, R.; Katsoulis, J. Radiographic bone level changes of implant-supported restorations in edentulous and partially dentate patients: 5-year results. Int. J. Oral Maxillofac. Implants 2014, 29, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Besimo, D.; Bodenschatz, V.; Guggenheim, R.; Hassell, T. Marginal fit of prefabricated crowns of the Ha-Ti implant system: An in vitro scanning electron microscopic study. Int. J. Prosthodont. 1996, 9, 87–94. [Google Scholar] [PubMed]
- Scarano, A.; Assenza, B.; Piattelli, M.; Iezzi, G.; Leghissa, G.C.; Quaranta, A.; Tortora, P.; Piattelli, A. A 16-year study of the microgap between 272 human titanium implants and their abutments. J. Oral Implantol. 2005, 31, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Assenza, B.; Artese, L.; Scarano, A.; Rubini, C.; Perrotti, V.; Piattelli, M.; Thams, U.; San Roman, F.; Piccirilli, M.; Piattelli, A. Screw vs cement-implant-retained restorations: An experimental study in the beagle. Part 2. Immunohistochemical evaluation of the peri-implant tissues. J. Oral Implantol. 2006, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaquiéry, C.; Ilgenstein, B.; Jungo, M.; Rüeger, K.; Chenaux, S.; Papadimitropoulos, A.; Jäger, K. Clinical and Radiological Outcome of Titanium Implants in Clinical Practice: A 5 Year, Prospective, Multicenter Case Series. Dent. J. 2014, 2, 106-117. https://doi.org/10.3390/dj2040106
Jaquiéry C, Ilgenstein B, Jungo M, Rüeger K, Chenaux S, Papadimitropoulos A, Jäger K. Clinical and Radiological Outcome of Titanium Implants in Clinical Practice: A 5 Year, Prospective, Multicenter Case Series. Dentistry Journal. 2014; 2(4):106-117. https://doi.org/10.3390/dj2040106
Chicago/Turabian StyleJaquiéry, Claude, Bernd Ilgenstein, Markus Jungo, Konrad Rüeger, Stephan Chenaux, Adam Papadimitropoulos, and Kurt Jäger. 2014. "Clinical and Radiological Outcome of Titanium Implants in Clinical Practice: A 5 Year, Prospective, Multicenter Case Series" Dentistry Journal 2, no. 4: 106-117. https://doi.org/10.3390/dj2040106
APA StyleJaquiéry, C., Ilgenstein, B., Jungo, M., Rüeger, K., Chenaux, S., Papadimitropoulos, A., & Jäger, K. (2014). Clinical and Radiological Outcome of Titanium Implants in Clinical Practice: A 5 Year, Prospective, Multicenter Case Series. Dentistry Journal, 2(4), 106-117. https://doi.org/10.3390/dj2040106




