In Situ Eradication of Mature Oral Biofilm on Titanium Implant Surfaces Using Cold Atmospheric Plasma
Abstract
1. Introduction
2. Materials and Methods
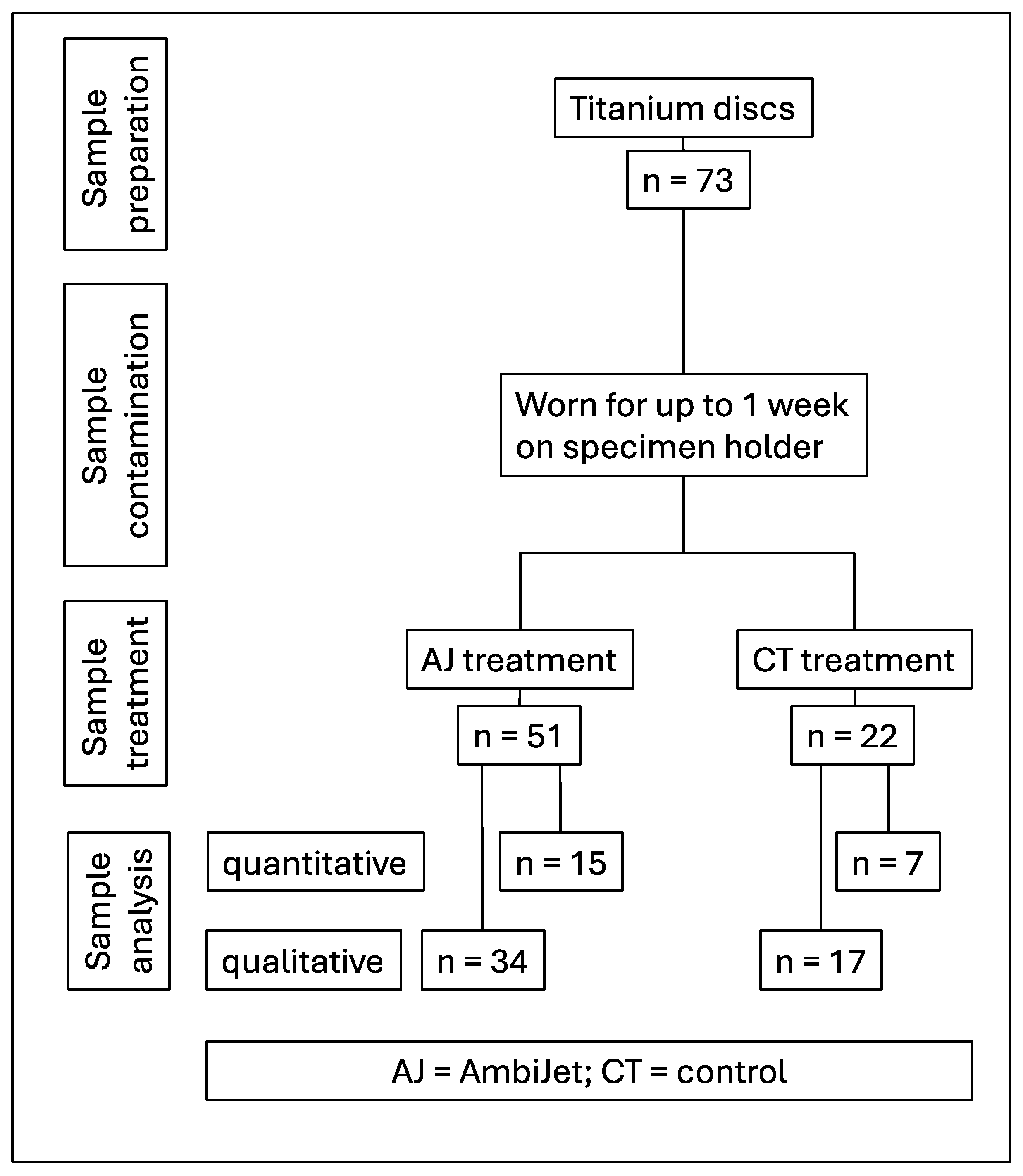
2.1. Study Design
2.2. Subject Selection
- Subjects must have an age between 18 and 60 years.
- No general illnesses relevant to the study.
- Subjects must have at least 20 teeth and acceptable oral hygiene (brushing at least twice a day).
- Able and willing to give written consent to participate in the study.
- Healthy or conservatively, prosthetically, and periodontally restored dentition.
- Willingness not to use antibacterial oral hygiene products (stannous fluoride, CHX, triclosan) during the study period.
- The salivary flow rate of the stimulated saliva should be at least 0.7 mL/min (determined by collecting saliva for one minute while chewing on a piece of paraffin).
- Dental treatments or other medical treatments in the oral cavity during the study period.
- Known allergies to previously used oral hygiene products and/or therapeutic products and/or dental materials used in the mouth or throat.
- Non-physiological tooth mobility.
- Pathological changes in the oral mucosa or gingiva.
- Excessive plaque formation.
- Eating disorders such as bulimia or anorexia nervosa.
- Use of antibiotics or chemical plaque treatments such as mouth rinses or varnishes
- Pregnancy or breastfeeding.
- Consumption of alcoholic beverages with more than 10% alcohol by volume (in their pure form or as a mixed drink).
- Past or current risky or pathological alcohol consumption—less than 2 alcohol-free days per week and/or more than one standard glass for women or 2 standard glasses for men of alcoholic beverages per day—as recommended by the government BZgA (Bundeszentrale für gesundheitliche Aufklärung).
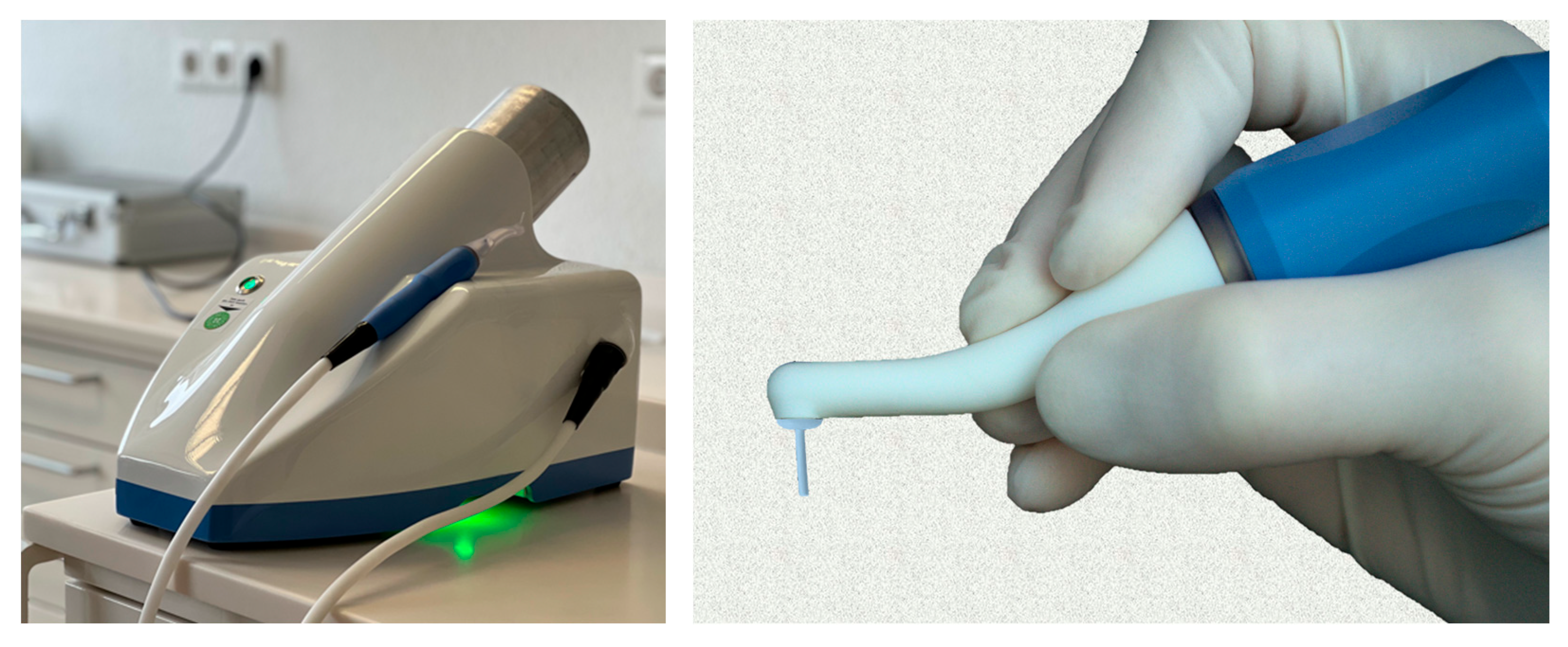
2.3. Plasma Device
2.4. Treatment
2.5. Microbial Analysis
- After treatment, wettened paper points (using tryptic soy broth, TSB) were used to wipe the treated or reference surfaces to collect bacteria off the surfaces.
- The paper points were transferred to Eppendorf tubes containing TSB and vortexed for 10 s.
- A dilution series was prepared for the CFU counting.
- A defined volume of the respective solutions was applied on agar plates and spatulated on Müller–Hinton agar in Petri dishes.
- The Petri dishes were incubated at 37 °C for two days to allow the bacteria to form colonies.
- The CFUs were then counted. The more of these CFUs were counted, the more bacteria were present in the detection solution and consequently in the samples, which indicates that there was a higher bacterial load on the samples.
2.6. Mathematical Analysis
3. Results
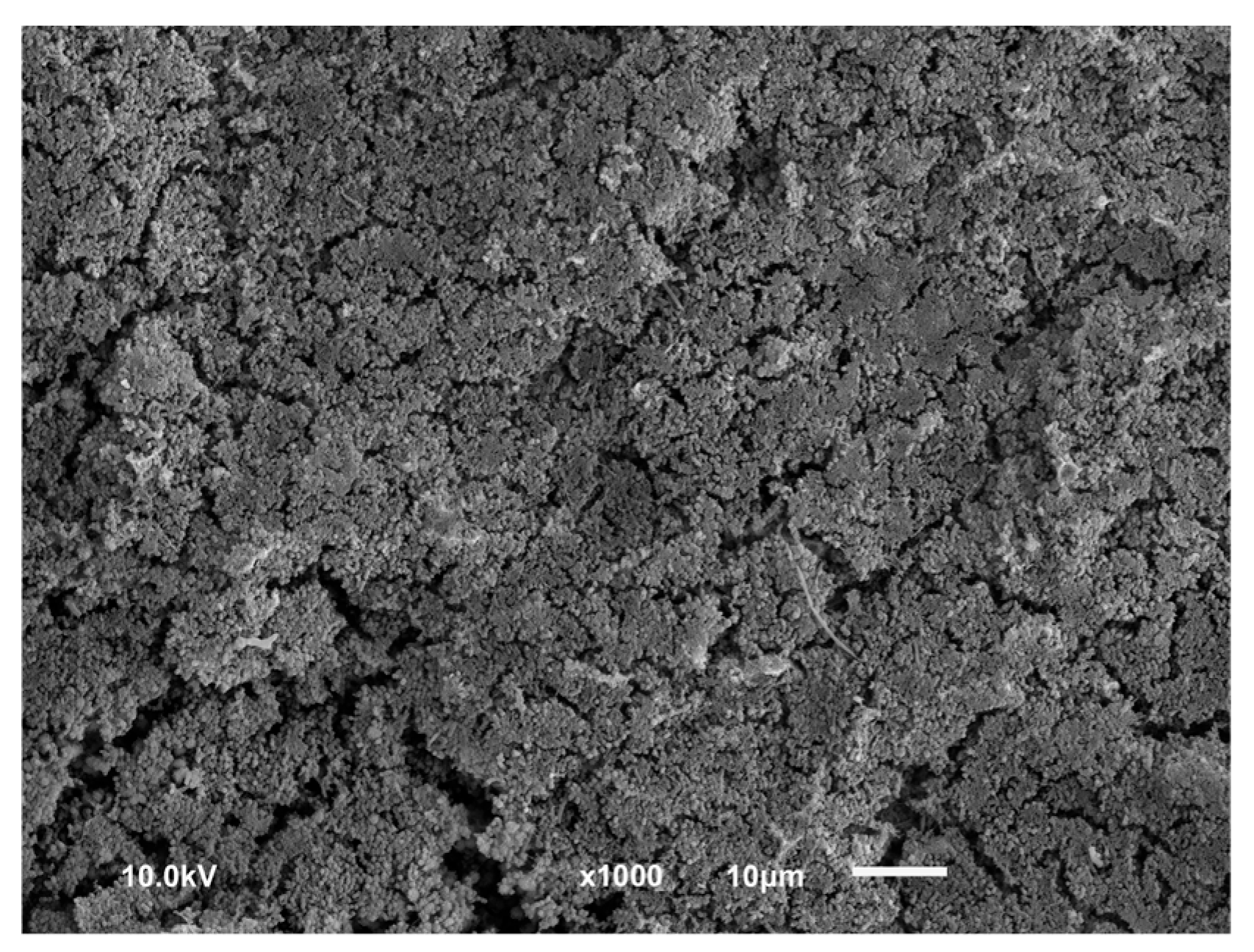
3.1. Qualitative Results
3.2. Quantitative Results
- The first quartile is long because 2 disks out of 34 delivered lower values.
- Out of 34 treated disks, 10 samples (ca. 30%) displayed a bacterial reduction ranging from 6-log(10) to 7-log(10). This is only obtained with control disk with a very high initial contamination.
- The samples displaying a ca. 4-log(10) reduction are bacteria-free. The bacterial reduction is lower because the initial contamination was lower for those splints.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Peri-implantitis—Onset and pattern of progression. J. Clin. Periodontol. 2016, 43, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Cecchinato, D.; Parpaiola, A.; Lindhe, J. Mucosal inflammation and incidence of crestal bone loss among implant patients: A 10-year study. Clin. Oral. Implants Res. 2014, 25, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Marrone, A.; Lasserre, J.; Bercy, P.; Brecx, M.C. Prevalence and risk factors for peri-implant disease in Belgian adults. Clin. Oral. Implants Res. 2013, 24, 934–940. [Google Scholar] [CrossRef]
- Ledernez, L.A.; Bergmann, M.E.; Altenburger, M.J. Periimplantitis, periodontitis, endodontics: A dental market analysis and future trends. J. Oral Health Oral Epidemiol. 2023, 12, 1–7. [Google Scholar] [CrossRef]
- Lin, H.Y.; Liu, Y.; Wismeijer, D.; Crielaard, W.; Deng, D.M. Effects of oral implant surface roughness on bacterial biofilm formation and treatment efficacy. Int. J. Oral Maxillofac. Implants 2013, 28, 1226–1231. [Google Scholar] [CrossRef]
- Harrel, S.K.; Wilson, T.G.; Pandya, M.; Diekwisch, T.G.H. Titanium particles generated during ultrasonic scaling of implants. J. Periodontol. 2019, 90, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, C.; Lusuardi, D.; Battarra, F.; Sassatelli, P.; Spinato, S.; Zaffe, D. The maintenance of inserted titanium implants: In-vitro evaluation of exposed surfaces cleaned with three different instruments. Clin. Oral Implants Res. 2017, 28, 57–63. [Google Scholar] [CrossRef]
- Yen Nee, W.; Raja Awang, R.A.; Hassan, A. Effects on the Titanium Implant Surface by Different Hygiene Instrumentations: A Narrative Review. Cureus 2022, 14, e30884. [Google Scholar] [CrossRef]
- Chen, L.; Tong, Z.; Luo, H.; Qu, Y.; Gu, X.; Si, M. Titanium particles in peri-implantitis: Distribution, pathogenesis and prospects. Int. J. Oral. Sci. 2023, 15, 49. [Google Scholar] [CrossRef]
- Pettersson, M.; Pettersson, J.; Johansson, A.; Molin Thorén, M. Titanium release in peri-implantitis. J. Oral Rehabil. 2019, 46, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Yu-Chieh Kao, A. Effect of Mechanical Decontamination Procedures on Moderately Roughened Titanium Surfaces: Quantity and Size of the Titanium Particulate Released by Mechanical Instrumentation; University of Otago: Dunedin, New Zealand, 2021. [Google Scholar]
- Ho, C.C.K. Biological Complications. In Practical Procedures in Implant Dentistry; Wiley: Hoboken, NJ, USA, 2021; pp. 351–369. [Google Scholar]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Lan, W.C.; Lan, W.H.; Chan, C.P.; Hsieh, C.C.; Chang, M.C.; Jeng, J.H. The effects of extracellular citric acid acidosis on the viability, cellular adhesion capacity and protein synthesis of cultured human gingival fibroblasts. Aust. Dent. J. 1999, 44, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Sawada, K.; Nakahara, K.; Haga-Tsujimura, M.; Fujioka-Kobayashi, M.; Iizuka, T.; Miron, R.J. Effect of Irrigation Time of Antiseptic Solutions on Bone Cell Viability and Growth Factor Release. J. Craniofac. Surg. 2018, 29, 376–381. [Google Scholar] [CrossRef]
- Carmeli, Y.; Troillet, N.; Karchmer, A.W.; Samore, M.H. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med. 1999, 159, 1127–1132. [Google Scholar] [CrossRef]
- Carmeli, Y.; Troillet, N.; Eliopoulos, G.M.; Samore, M.H. Emergence of antibiotic-resistant Pseudomonas aeruginosa: Comparison of risks associated with different antipseudomonal agents. Antimicrob. Agents Chemother. 1999, 43, 1379–1382. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic Therapy in Dentistry. Int. J. Dent. 2021, 2021, 6667624. [Google Scholar] [CrossRef]
- Sukumar, S.; Martin, F.E.; Hughes, T.E.; Adler, C.J. Think before you prescribe: How dentistry contributes to antibiotic resistance. Aust. Dent. J. 2020, 65, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Williams, D.; Pulcini, C.; Sanderson, S.; Calfon, P.; Verma, M. Tackling Antibiotic Resistance: Why Dentistry Matters. Int. Dent. J. 2021, 71, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Farmer, J.; Singhal, S.; Marra, F.; Sutherland, S.; Quiñonez, C. The use and misuse of antibiotics in dentistry: A scoping review. J. Am. Dent. Assoc. 2018, 149, 869–884.e5. [Google Scholar] [CrossRef]
- Al-Haroni, M. Bacterial resistance and the dental professionals’ role to halt the problem. J. Dent. 2008, 36, 95–103. [Google Scholar] [CrossRef]
- Fridman, A.; Friedman, G. Plasma Medicine; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Metelmann, H.-R.; von Woedtke, T.; Weltmann, K.-D. Plasmamedizin; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Wu, S.; Cao, Y.; Lu, X. The State of the Art of Applications of Atmospheric-Pressure Nonequilibrium Plasma Jets in Dentistry. IEEE Trans. Plasma Sci. 2016, 44, 134–151. [Google Scholar] [CrossRef]
- Gherardi, M.; Tonini, R.; Colombo, V. Plasma in Dentistry: Brief History and Current Status. Trends Biotechnol. 2018, 36, 583–585. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Zhou, X.; Liu, Z.; Wang, C.; Wang, K.; Zhang, J.; Wang, Z. A novel cold atmospheric pressure air plasma jet for peri-implantitis treatment: An in vitro study. Dent. Mater. J. 2018, 37, 157–166. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, E.-H.; Kim, K.-M.; Kim, K.-N. Effect of non-thermal air atmospheric pressure plasma jet treatment on gingival wound healing. J. Phys. D Appl. Phys. 2016, 49, 075402. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeong, W.S.; Seo, S.J.; Kim, H.W.; Kim, K.N.; Choi, E.H.; Kim, K.M. Non-thermal atmospheric pressure plasma functionalized dental implant for enhancement of bacterial resistance and osseointegration. Dent. Mater. 2017, 33, 257–270. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, J.S.; Jiang, H.B.; Choi, E.H.; Park, G.; Kim, K.M. The antibacterial effect of non-thermal atmospheric pressure plasma treatment of titanium surfaces according to the bacterial wall structure. Sci. Rep. 2019, 9, 1938. [Google Scholar] [CrossRef]
- Duarte, S.; Panariello, B.H.D. Comprehensive biomedical applications of low temperature plasmas. Arch. Biochem. Biophys. 2020, 693, 108560. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.H.; Choi, E.H.; Kim, K.M.; Kim, K.N. Air atmospheric-pressure plasma-jet treatment enhances the attachment of human gingival fibroblasts for early peri-implant soft tissue seals on titanium dental implant abutments. Acta Odontol. Scand. 2015, 73, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Eggers, B.; Duddeck, D.; Kramer, F.-J.; Bourauel, C.; Jepsen, S.; Deschner, J.; Nokhbehsaim, M. Influence of cold atmospheric plasma on dental implant materials—An in vitro analysis. Clin. Oral. Investig. 2022, 26, 2949–2963. [Google Scholar] [CrossRef]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Duske, K.; Jablonowski, L.; Koban, I.; Matthes, R.; Holtfreter, B.; Sckell, A.; Nebe, J.B.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Cold atmospheric plasma in combination with mechanical treatment improves osteoblast growth on biofilm covered titanium discs. Biomaterials 2015, 52, 327–334. [Google Scholar] [CrossRef]
- Preissner, S.; Wirtz, H.C.; Tietz, A.-K.; Abu-Sirhan, S.; Herbst, S.R.; Hartwig, S.; Pierdzioch, P.; Schmidt-Westhausen, A.M.; Dommisch, H.; Hertel, M. Bactericidal efficacy of tissue tolerable plasma on microrough titanium dental implants: Anin-vitro-study. J. Biophotonics 2016, 9, 637–644. [Google Scholar] [CrossRef]
- Piest, C.; Wille, S.; Strunskus, T.; Polonskyi, O.; Kern, M. Efficacy of Plasma Treatment for Decontaminating Zirconia. J. Adhes. Dent. 2018, 20, 289–298. [Google Scholar]
- Hui, W.L.; Perrotti, V.; Piattelli, A.; Ostrikov, K.; Fang, Z.; Quaranta, A. Cold atmospheric plasma coupled with air abrasion in liquid medium for the treatment of peri-implantitis model grown with a complex human biofilm: An in vitro study. Clin. Oral. Investig. 2021, 25, 6633–6642. [Google Scholar] [CrossRef]
- Gross, T.; Ledernez, L.A.; Birrer, L.; Bergmann, M.E.; Altenburger, M.J. Guided Plasma Application in Dentistry—An Alternative to Antibiotic Therapy. Antibiotics 2024, 13, 735. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Faust, J.; Bächle, M.; Follo, M.; Wolkewitz, M.; Hannig, C.; Hellwig, E.; Carvalho, C.; Kohal, R. Biofilm formation and composition on different implant materials in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95B, 101–109. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wunder, A.; Auschill, T.M.; Follo, M.; Braun, G.; Hellwig, E.; Arweiler, N.B. The in vivo dynamics of Streptococcus spp., Actinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. J. Med. Microbiol. 2007, 56, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Follo, M.; Hellwig, E.; Burghardt, D.; Wolkewitz, M.; Anderson, A.; Al-Ahmad, A. Microscope-Based Imaging Platform for Large-Scale Analysis of Oral Biofilms. Appl. Environ. Microbiol. 2012, 78, 8703–8711. [Google Scholar] [CrossRef] [PubMed]




| After 24 h | After 48 h | After 72 h | ||
|---|---|---|---|---|
| Plasma group | Negative (no bacteria) | 14 | 14 | 14 |
| Positive (contamination) | 1 | 1 | 1 | |
| Control group | Negative (no bacteria) | 0 | 0 | 0 |
| Positive (contamination) | 7 | 7 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altenburger, M.J.; Bergmann, M.E.; Ledernez, L.A.; Romanos, G. In Situ Eradication of Mature Oral Biofilm on Titanium Implant Surfaces Using Cold Atmospheric Plasma. Dent. J. 2025, 13, 210. https://doi.org/10.3390/dj13050210
Altenburger MJ, Bergmann ME, Ledernez LA, Romanos G. In Situ Eradication of Mature Oral Biofilm on Titanium Implant Surfaces Using Cold Atmospheric Plasma. Dentistry Journal. 2025; 13(5):210. https://doi.org/10.3390/dj13050210
Chicago/Turabian StyleAltenburger, Markus Jörg, Michael Eckhard Bergmann, Loic Alain Ledernez, and Georgios Romanos. 2025. "In Situ Eradication of Mature Oral Biofilm on Titanium Implant Surfaces Using Cold Atmospheric Plasma" Dentistry Journal 13, no. 5: 210. https://doi.org/10.3390/dj13050210
APA StyleAltenburger, M. J., Bergmann, M. E., Ledernez, L. A., & Romanos, G. (2025). In Situ Eradication of Mature Oral Biofilm on Titanium Implant Surfaces Using Cold Atmospheric Plasma. Dentistry Journal, 13(5), 210. https://doi.org/10.3390/dj13050210






