SEM and Bacteriological Evidence of Laser-Activated Irrigation Compared to Ultrasonic-Activated Irrigation: A Pilot Study
Abstract
1. Introduction
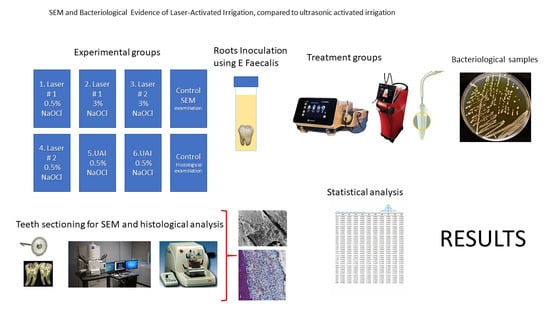
2. Material and Methods
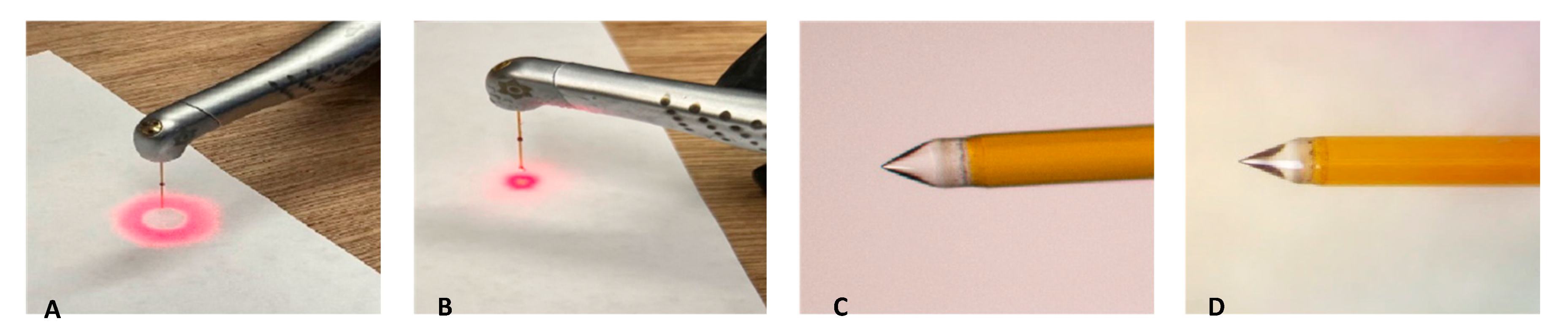
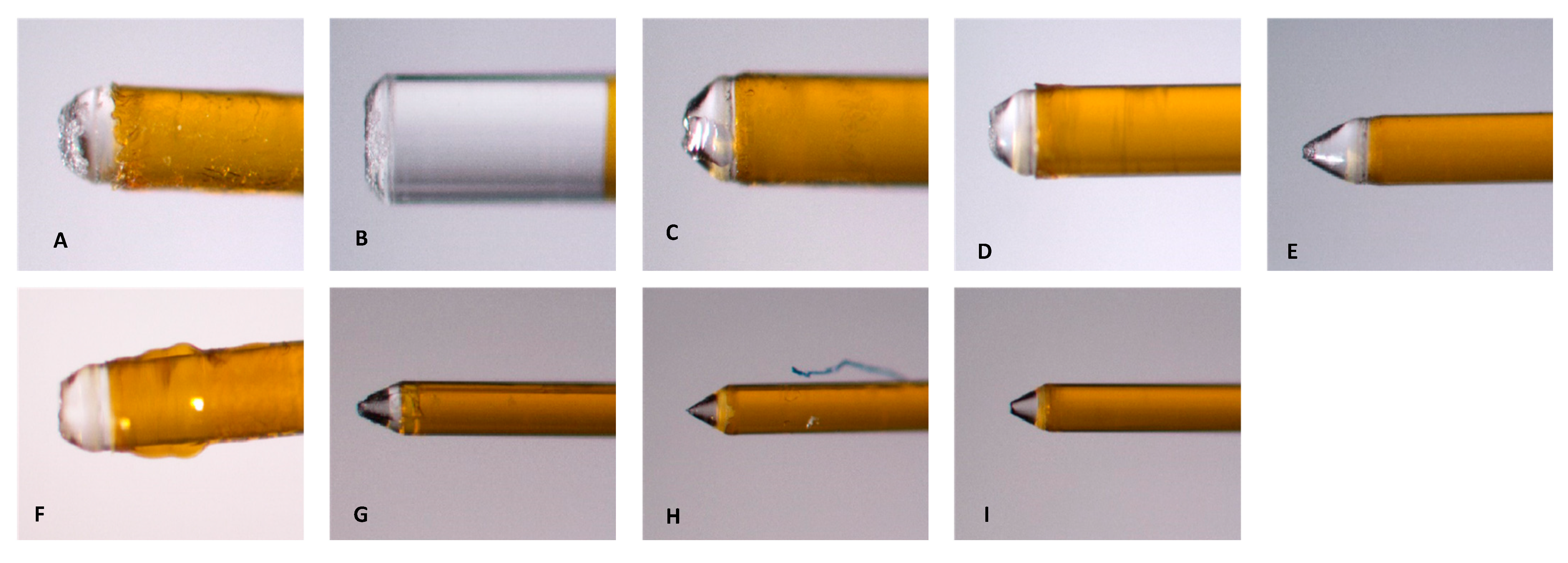
2.1. Preparation of Laser Systems
2.2. Laser-Activated Irrigation (LAI) Protocols
3. Bacteriological Samples
3.1. CFU Bacterial Analysis
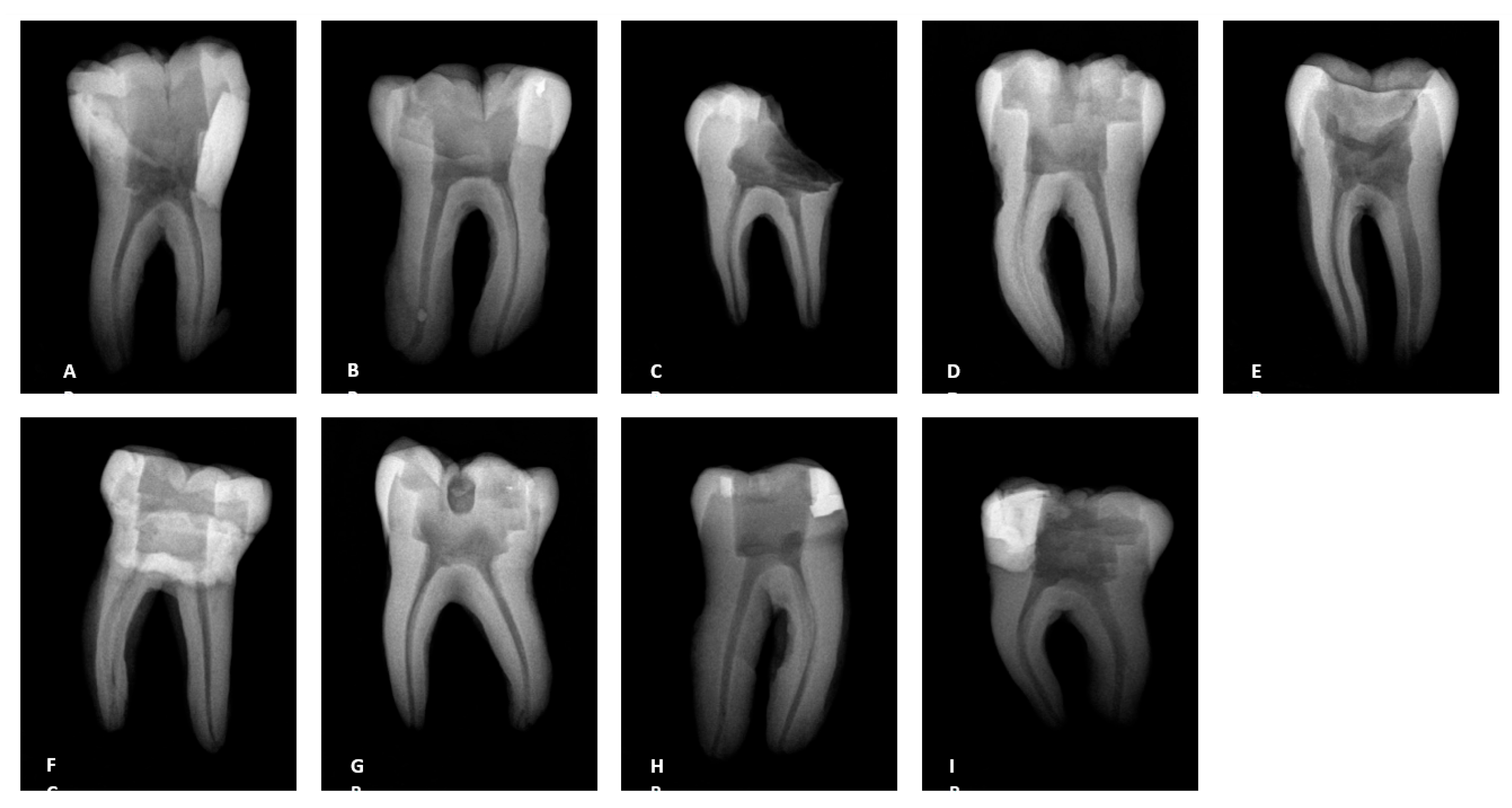
3.2. Radiographs
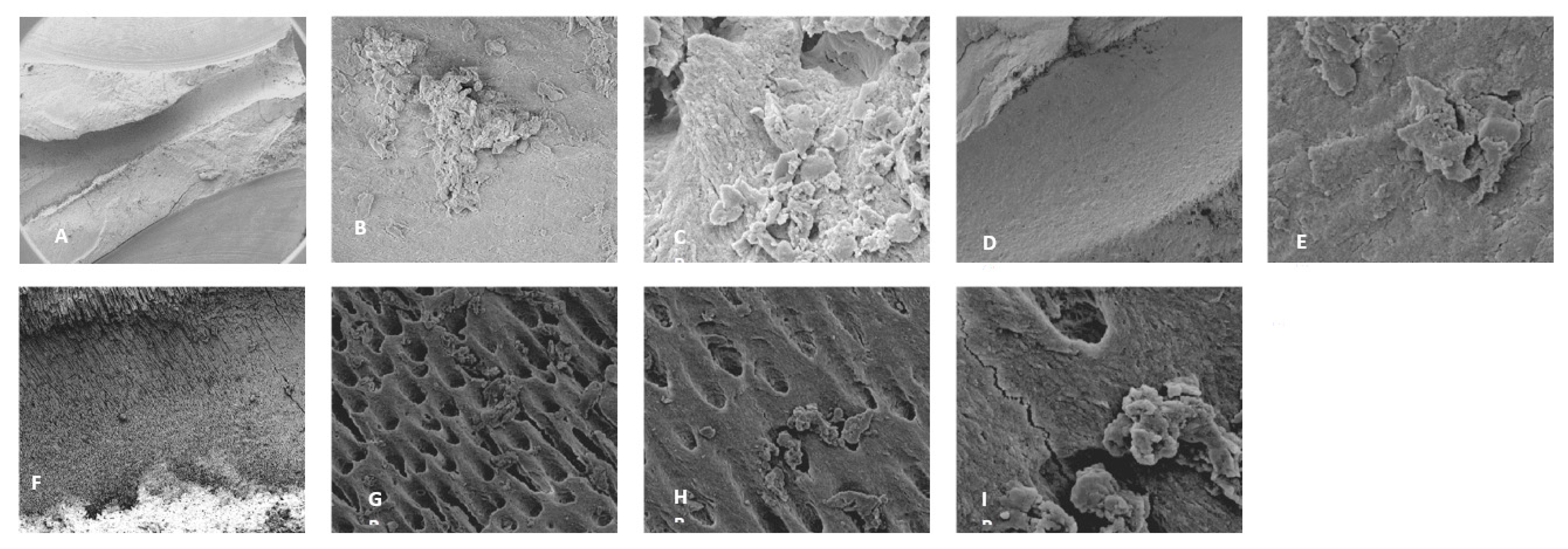
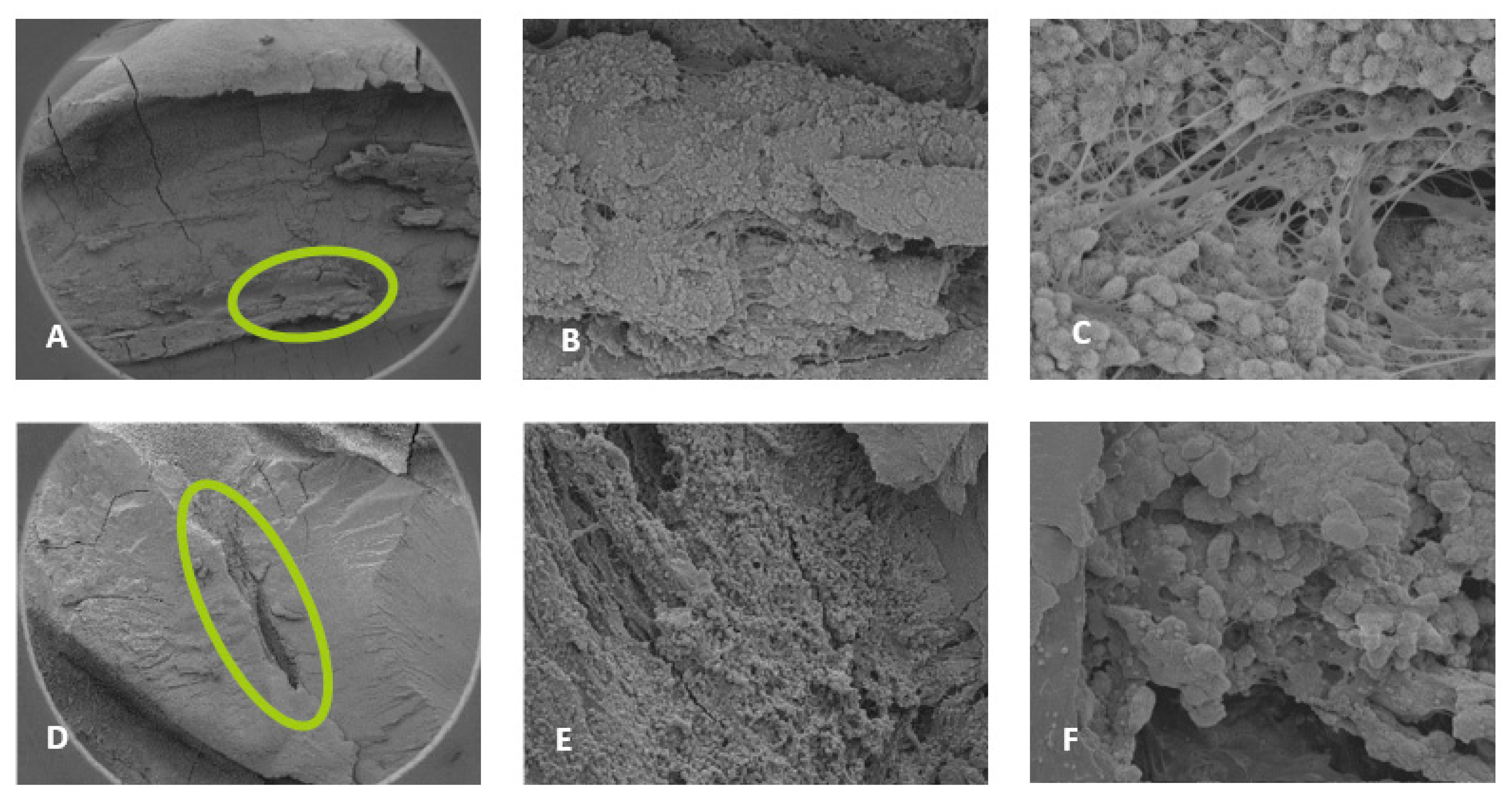
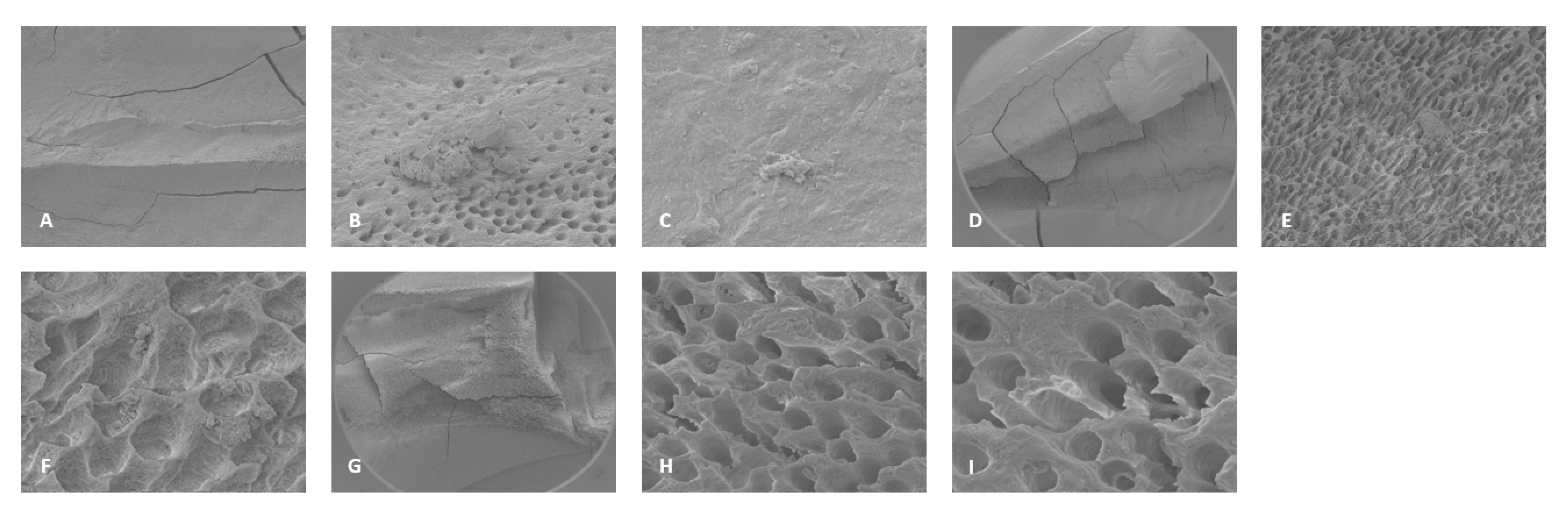
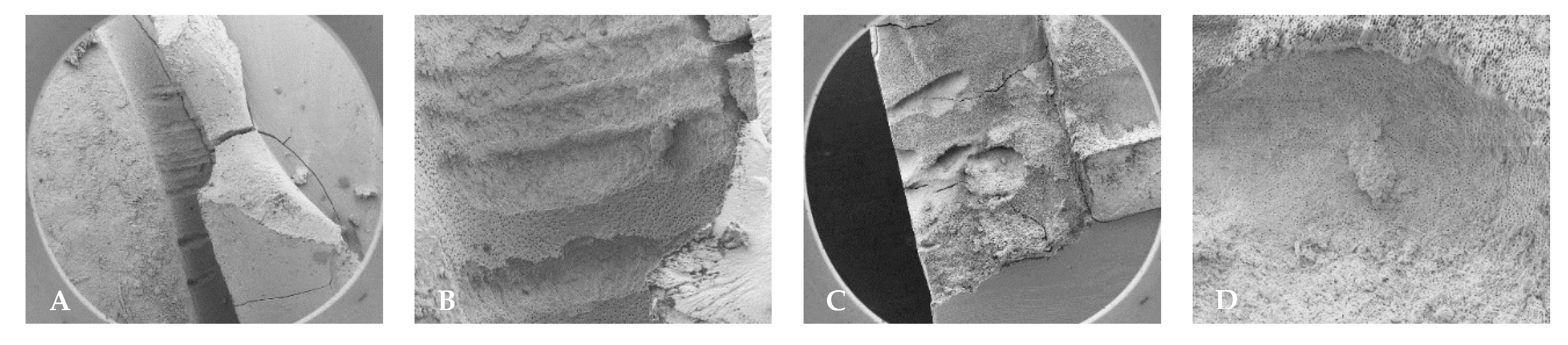
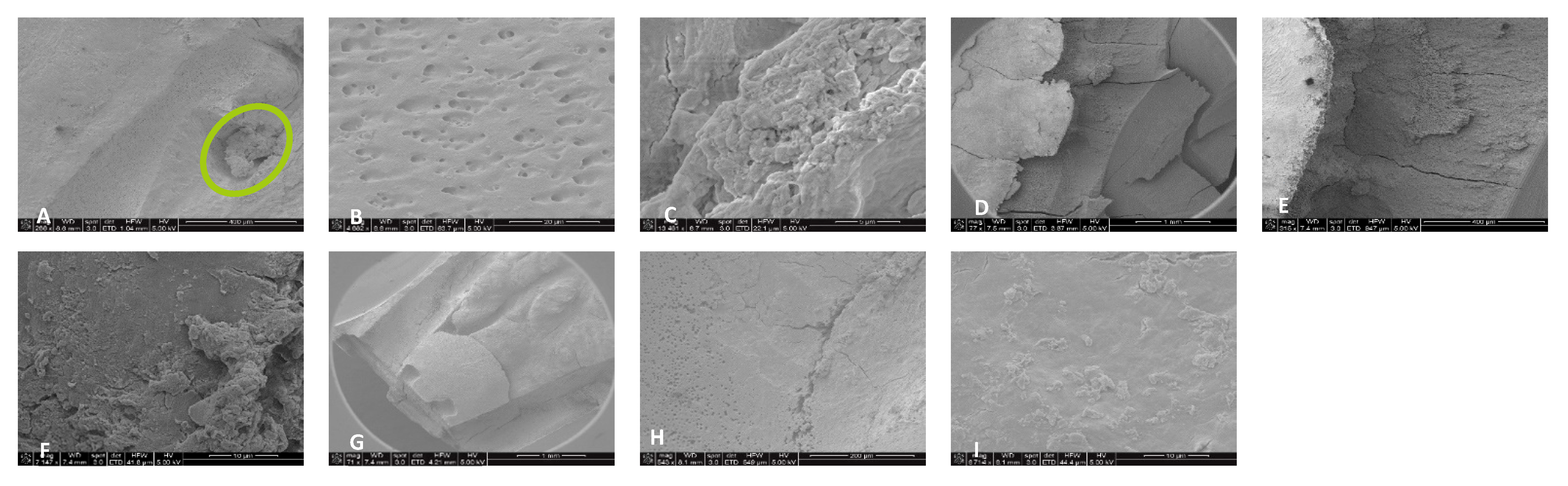
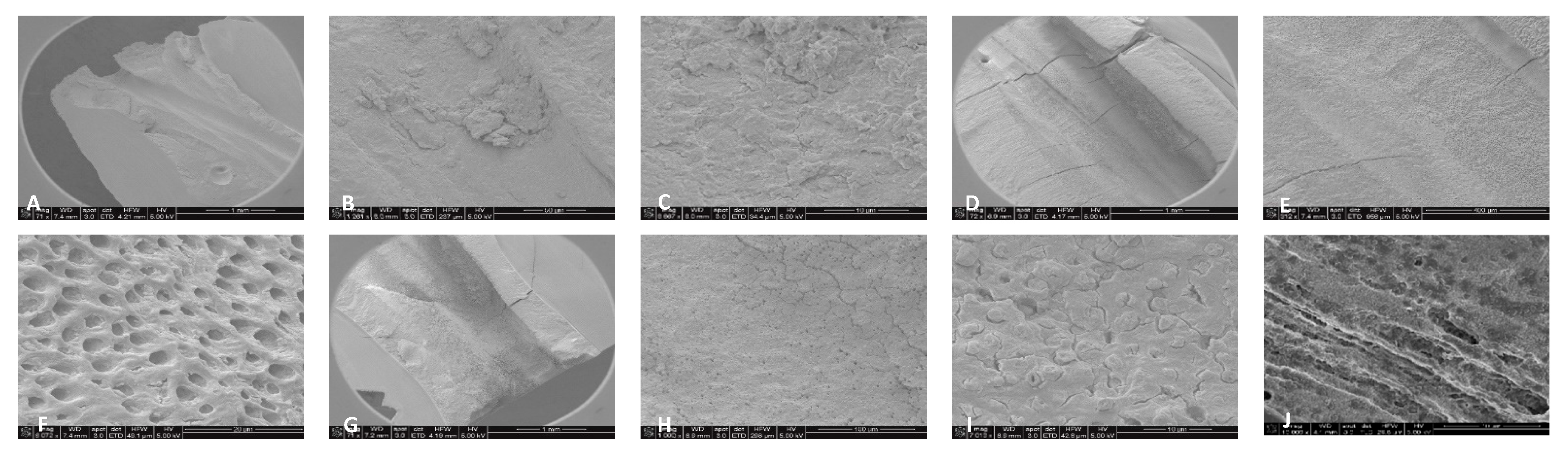
3.3. Scanning Electron Microscopy (SEM)
3.4. Stereomicroscope Images
3.5. Statistical Analysis
4. Results
4.1. Bacteriological Samples
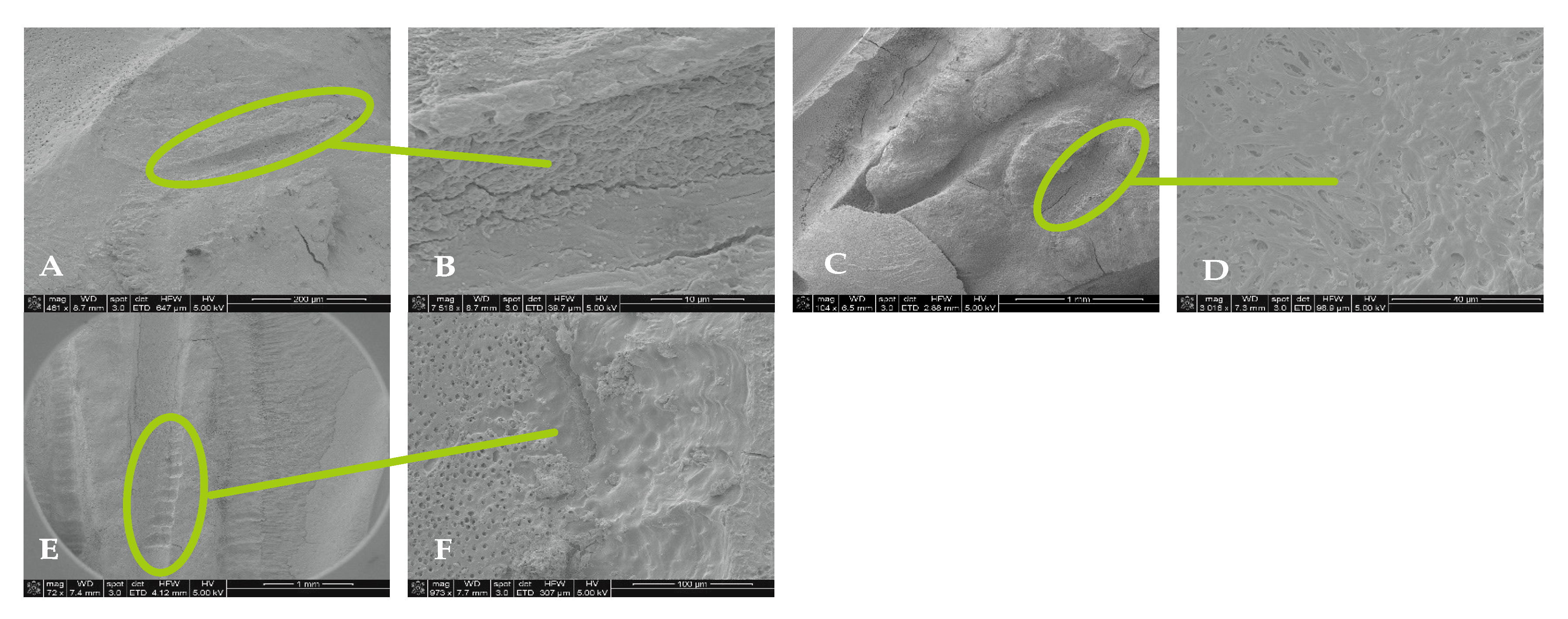
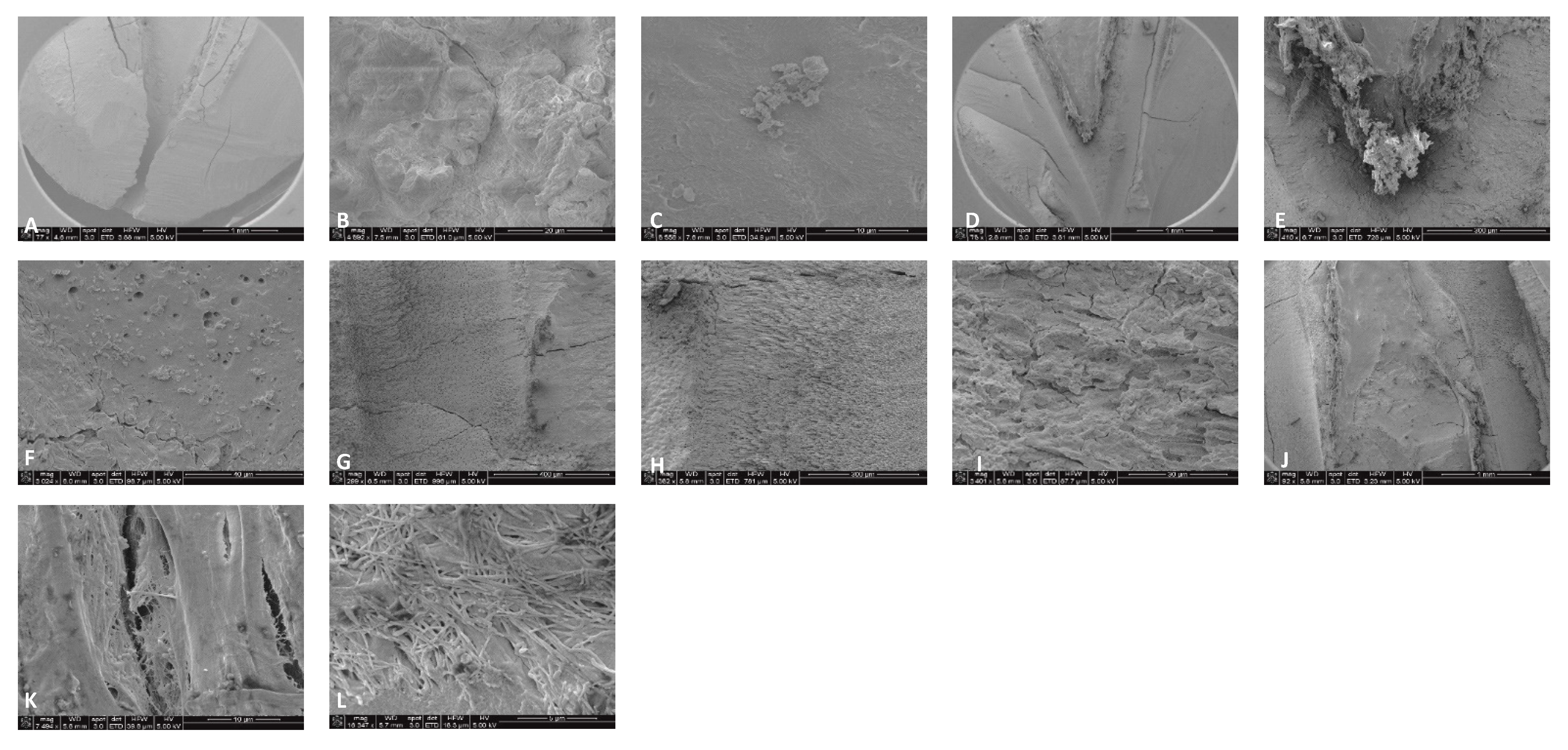
4.2. SEM Analyses
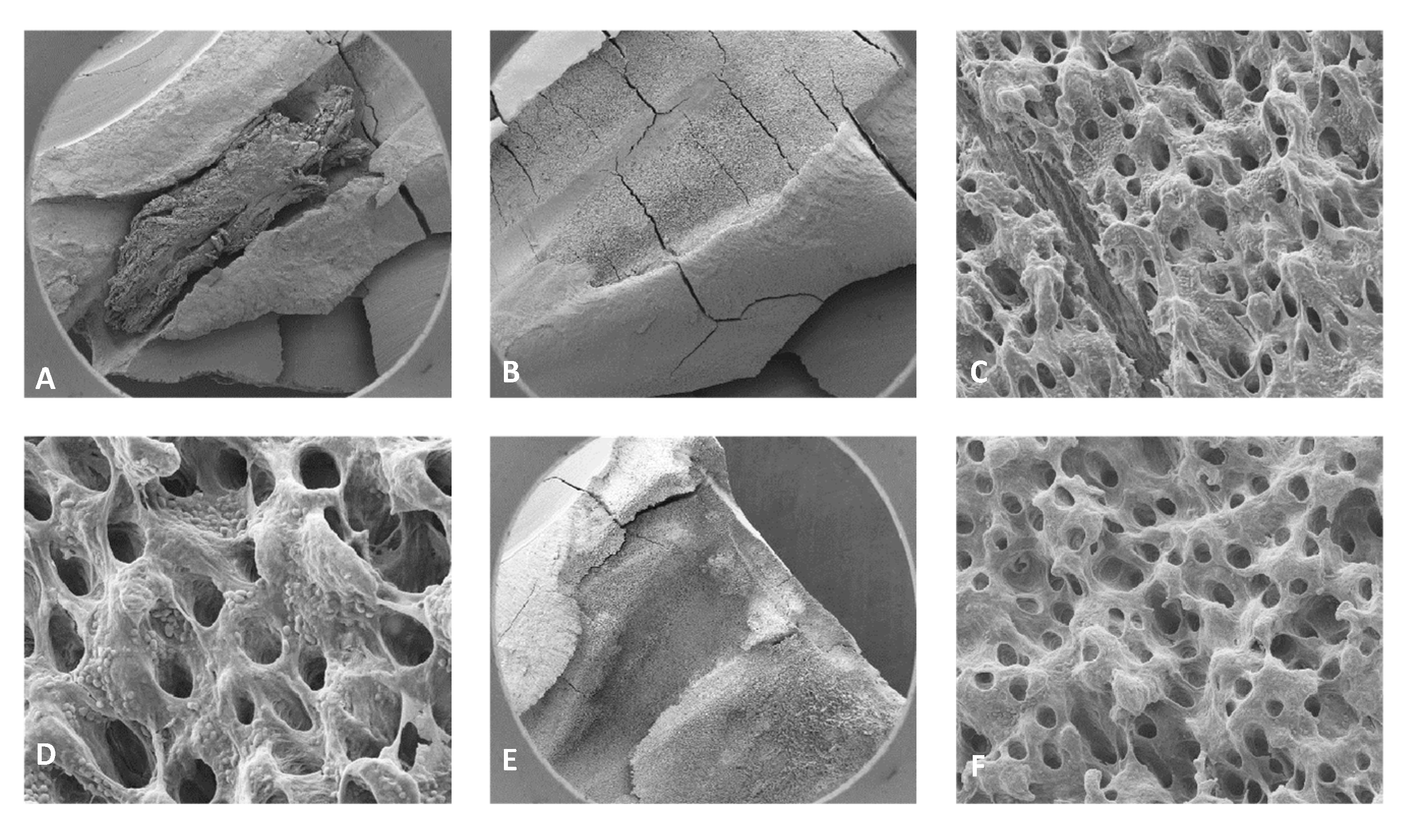
4.3. Stereomicroscope Images
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meire, M.A.; De Prijck, K.; Coenye, T.; Nelis, H.J.; De Moor, R.J.G. Effectiveness of different laser systems to kill Enterococcus faecalis in aqueous suspension and in an infected tooth model. Int. Endod. J. 2009, 42, 351–359. [Google Scholar] [CrossRef]
- de Paz, L.E.C.; Bergenholtz, G.; Svensäter, G. The effects of antimicrobials on endodontic biofilm bacteria. J. Endod. 2010, 36, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Noetzel, J.; Nonhoff, J.; Bitter, K.; Wagner, J.; Neumann, K.; Kielbassa, A.M. Efficacy of calcium hydroxide. Er: YAG laser or gaseous ozone against Enterococcus faecalis in root canals. Am. J. Dent. 2009, 22, 14–18. [Google Scholar] [PubMed]
- Dewsnup, N.; Pileggi, R.; Haddix, J.; Nair, U.; Walker, C.; Varella, C.H. Comparison of bacterial reduction in straight and curved canals using erbium, chromium:yttrium-scandium-gallium-garnet laser treatment versus a traditional irrigation technique with sodium hypochlorite. J. Endod. 2010, 36, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Franzen, R.; Gutknecht, N.; Falken, S.; Heussen, N.; Meister, J. Bactericidal effect of an Nd:YAG laser on Enterococcus faecalis at pulse durations of 15 and 25 ms in dentine depths of 500 and 1000 PM. Lasers Med. Sci. 2011, 26, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z. Chemo mechanical strategies to manage endodontic infections. Dent. Today 2010, 29, 91–92, 94, 96. [Google Scholar] [PubMed]
- Upadya, M.H.; Kishen, A. Influence of bacterial growth modes on the susceptibility to light-activated disinfection. Int. Endod. J. 2010, 43, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.; Boone, M.; De Bruyne, M.; De Moor, R.; Versani, M.A.; Meire, M. Adjunctive steps for the removal of hard tissue debris from the anatomic complexities of the mesial root canal system of mandibular molars: A micro-computed tomographic study. J. Endod. 2020, 46, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Kayaoglu, G.; Orstavik, D. Virulence factors of Enterococcus faecalis: Relationship to endodontic disease. Crit. Rev. Oral Biol. Med. 2004, 15, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Stojicic, S.; Zivkovic, S.; Quan, W.; Zhang, H.; Haapasalo, M. Tissue dissolution by sodium hypochlorite: Effect of concentration, temperature, agitation, and surfactant. J. Endod. 2010, 3, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- De Moor, R.J.; Meir, M.; Goharkhay, K.; Moritz, A.; Vanobbergen, J. Efficacy of ultrasonic versus laser-activated irrigation to remove artificially placed dentin debris plugs. J. Endod. 2010, 36, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, W.T.; Martin, H.; Forrest, W.R. Evaluation of root canal debridement by the endosonic ultrasonic synergistic system. Oral Surg. Oral Med. Oral Pathol. 1982, 53, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.W. A comparison f canal preparations in stright and curve canals. Oral Surg Oral Med. Oral Pathol. 1971, 32, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, P.; Merlos, A.; Sierra, J.M.; Arnabat-Dominguez, J.; Viñas, M. Er,Cr:YSGG Laser-Activated Irrigation and Passive Ultrasonic Irrigation: Comparison of Two Strategies for Root Canal Disinfection. Photobiomodul. Photomed. Laser Surg. 2020, 38, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Yavari, H.R.; Rahimi, S.; Shahi, S.; Lotfi, M.; Barhaghi, M.H.; Fatemi, A.; Abdolrahimi, M. Effect of Er, Cr: YSGG laser irradiation on Enterococcus faecalis in infected root canals. Photomed. Laser Surg. 2010, 28 (Suppl. 1), 591–596. [Google Scholar] [CrossRef] [PubMed]
- Kolnick, J. Laser 101. The Secret Life of Laser Dentist. Available online: https://podcasts.apple.com/us/podcast/dr-justin-kolnicks-simple-method-for-over-95-endodontic/id1675376834?i=1000607454554 (accessed on 15 April 2025).
- Li, Z.; Code, J.E.; Van De Merwe, W.P. Er: YAG Laser ablation of enamel and dentin of human teeth: Determination of the ablation rates at various fluences and pulse repetition rates. Lasers Surg. Med. 1992, 12, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Majaron, B.; Lukac, M.; Sustercic, D.; Funduk, N.; Skaleric, U. Threshold and efficiency analysis in Er: YAG laser ablation of hard dental tissue. In Laser Applications in Medicine and Dentistry; SPIE: Bellingham, WA, USA, 1996; Volume 2922, pp. 233–242. [Google Scholar]
- Home. EdgePro. 17 October 2022. Available online: https://www.edgeproendo.com/ (accessed on 19 October 2022).
- Maupin C TTP AAE 2020 Lecture, Dynamic Guidance and Lasers: Navigating Endodontics Into the Future? Ep. 10. Available online: https://www.aae.org/specialty/podcast/dynamic-guidance-and-lasers-navigating-endodontics-into-the-future-ep-10/ (accessed on 15 April 2025).
| Group | Technique | Irrigation Protocol |
|---|---|---|
| 1 | EdgePro | 0.5% Sodium hypochlorite |
| 2 | EdgePro | 3% Sodium hypochlorite + 17% EDTA |
| 3 | Waterlase | 3% Sodium hypochlorite + 17% EDTA |
| 4 | Waterlase | 0.5% Sodium hypochlorite |
| 5 | UAI | 0.5% Sodium hypochlorite |
| 6 | UAI | 3% Sodium hypochlorite |
| LR Chi sq | Df | Pr (>Chi sq) | |
|---|---|---|---|
| Irrigation | 1.48497 | 2 | 0.4759 |
| NaOCl | 0.05221 | 1 | 0.8193 |
| Irrigation: NaOCl | 0.49962 | 2 | 0.7789 |
| 0.5% | 3% | |
|---|---|---|
| EdgePro | 354,212.0 | 381,660.0 |
| UA | 328,920.8 | 44,959.4 |
| WaterLase | 487,995.4 | 584,121.4 |
| 0.5% | 3% | |
|---|---|---|
| EdgePro | 752,692.3 | 741,913.43 |
| UA | 500,120.5 | 39,708.67 |
| WaterLase | 598,095.4 | 858,527.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo, D.E.; Jeong, J.W.; Shen, Z.; Divito, E. SEM and Bacteriological Evidence of Laser-Activated Irrigation Compared to Ultrasonic-Activated Irrigation: A Pilot Study. Dent. J. 2025, 13, 195. https://doi.org/10.3390/dj13050195
Jaramillo DE, Jeong JW, Shen Z, Divito E. SEM and Bacteriological Evidence of Laser-Activated Irrigation Compared to Ultrasonic-Activated Irrigation: A Pilot Study. Dentistry Journal. 2025; 13(5):195. https://doi.org/10.3390/dj13050195
Chicago/Turabian StyleJaramillo, David E., Ji W. Jeong, Zhen Shen, and Enrico Divito. 2025. "SEM and Bacteriological Evidence of Laser-Activated Irrigation Compared to Ultrasonic-Activated Irrigation: A Pilot Study" Dentistry Journal 13, no. 5: 195. https://doi.org/10.3390/dj13050195
APA StyleJaramillo, D. E., Jeong, J. W., Shen, Z., & Divito, E. (2025). SEM and Bacteriological Evidence of Laser-Activated Irrigation Compared to Ultrasonic-Activated Irrigation: A Pilot Study. Dentistry Journal, 13(5), 195. https://doi.org/10.3390/dj13050195