Comparative Analysis of Salivary Tumor Marker CA-125 Among Oral Squamous Cell Carcinoma Patients and Healthy Individuals
Abstract
:1. Introduction
2. Materials and Methods
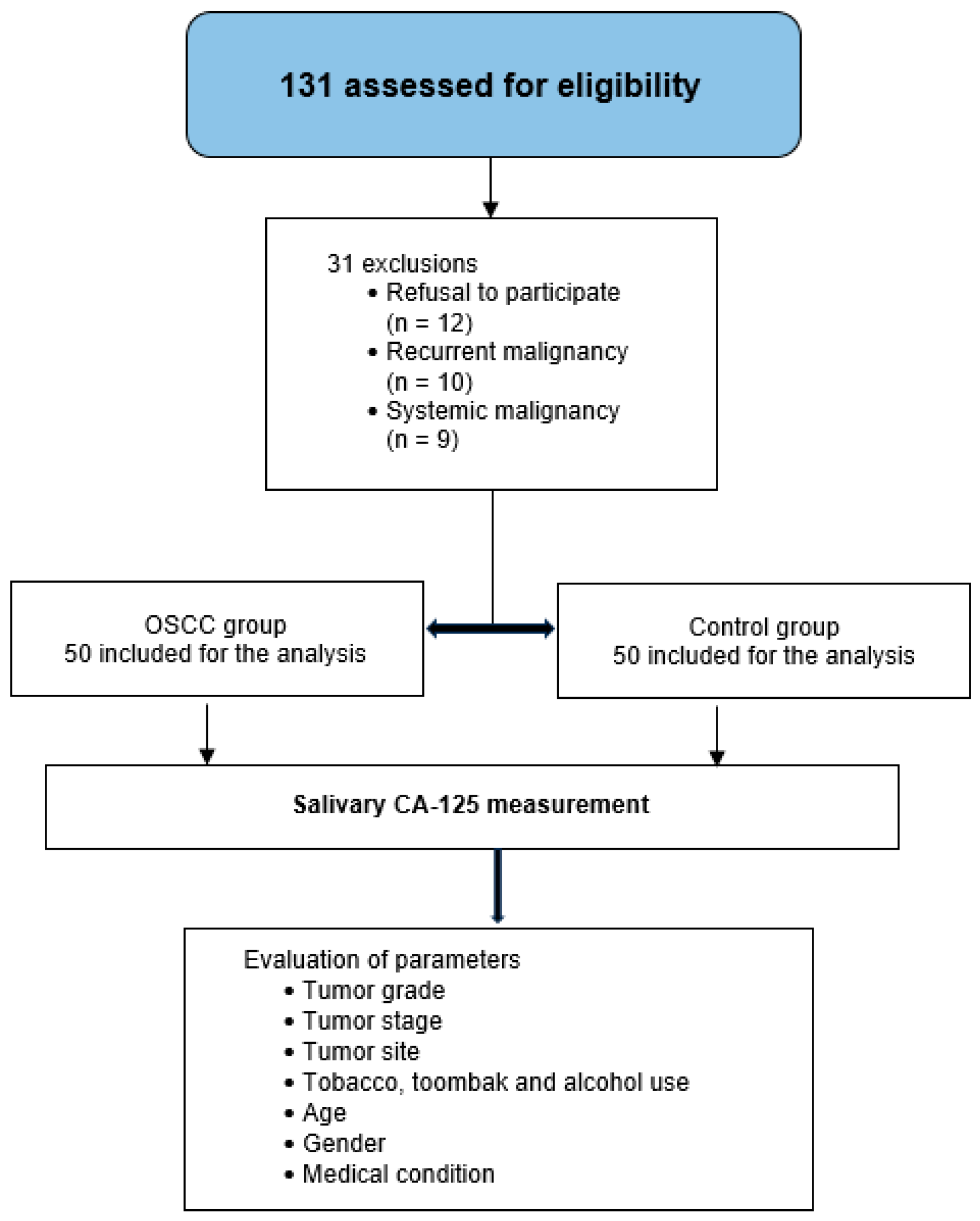
2.1. Selection of Cases and Healthy Participants
- Refusal to participate
- History of premalignant oral lesions or other forms of oral cancer
- Past or current diagnosis of systemic malignancies known to elevate CA-125 levels (e.g., ovarian, breast, endometrial, or colorectal cancers)
- Oral mucosal conditions with bleeding potential (e.g., acute gingivitis, traumatic ulcers)
- Xerostomia or salivary gland disorders affecting saliva quantity or quality
- Inability to provide a minimum of 3 mL of saliva
- Pregnant, lactating, or menstruating women (to control for hormonal variation in CA-125 levels)
2.2. Saliva Collection
2.3. Quantification of Salivary CA-125
2.4. Statistical Analysis
3. Results
3.1. Participant and Variable Distribution
3.2. Comparative and Correlational Analyses
3.3. Diagnostic Performance Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA-125 | Cancer antigen 125 |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FEIA | Fluorescent enzyme immunoassay |
| OC | Oral cancer |
| OSCC | Oral squamous cell carcinoma |
| rpm | Round per minute |
| U/ml | Units per milliliter |
References
- Ramnarayan, B.K.; Akhilesh, S.; Babu, H.M. An insight into salivary markers in oral cancer. Dent. Res. J. 2013, 10, 287–295. [Google Scholar]
- Wu, J.Y.; Yi, C.; Chung, H.R.; Wang, D.J.; Chang, W.C.; Lee, S.Y.; Lin, C.T.; Yang, Y.C.; Yang, W.C. Potential biomarkers in saliva for oral squamous cell carcinoma. Oral. Oncol. 2010, 46, 226–231. [Google Scholar] [CrossRef]
- WHO. International Statistical Classification of Diseases ICD-11 for Mortality and Morbidity Statistics, Version: 01/2023. Available online: https://icd.who.int/en (accessed on 23 March 2025).
- Gaafar, N.M.; Osman, T.A.; Elsheikh, M.; Ahmed, I.A.; Dongre, H.; Fromreide, S.; Suleiman, A.M.; Johannessen, A.C.; Nginamau, E.S.; Costea, D.E. Epithelial PD-L1 expression at tumor front predicts overall survival in a cohort of oral squamous cell carcinomas from Sudan. Clin. Exp. Dent. Res. 2022, 8, 1467–1477. [Google Scholar] [CrossRef]
- Federal Ministry of Health (Sudan). Sudan Annual Health Statistics Report 2012; Federal Ministry of Health (Sudan): Khartoum, Sudan, 2012.
- Markopoulos, A.K.; Michailidou, E.Z.; Tzimagiorgis, G. Salivary markers for oral cancer detection. Open Dent. J. 2010, 4, 172–178. [Google Scholar] [CrossRef]
- Balan, J.J.; Rao, R.S.; Premalatha, B.R.; Patil, S. Analysis of tumor marker CA 125 in saliva of normal and oral squamous cell carcinoma patients: A comparative study. J. Contemp. Dent. Pract. 2012, 13, 671–675. [Google Scholar] [CrossRef]
- Nagler, R.; Bahar, G.; Shpitzer, T.; Feinmesser, R. Concomitant analysis of salivary tumor markers--a new diagnostic tool for oral cancer. Clin. Cancer Res. 2006, 12, 3979–3984. [Google Scholar] [CrossRef] [PubMed]
- Rajguru, J.P.; Mouneshkumar, C.D.; Radhakrishnan, I.C.; Negi, B.S.; Maya, D.; Hajibabaei, S.; Rana, V. Tumor markers in oral cancer: A review. J. Fam. Med. Prim. Care 2020, 9, 492–496. [Google Scholar]
- Sanjay, R.B.; Madhavi, R.B.; Shyam, N. Tumor markers in oral neoplasia. IJDA 2010, 2, 103–114. [Google Scholar]
- Shaw, A.K.; Garcha, V.; Shetty, V.; Vinay, V.; Bhor, K.; Ambildhok, K.; Karande, P. Diagnostic Accuracy of Salivary Biomarkers in Detecting Early Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2022, 23, 1483–1495. [Google Scholar] [CrossRef]
- Yin, B.W.; Lloyd, K.O. Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. J. Biol. Chem. 2001, 276, 27371–27375. [Google Scholar] [CrossRef]
- Bhargava, A.; Saigal, S.; Chalishazar, M. Histopathological grading systems in oral squamous cell carcinoma: A review. J. Int. Oral. Health 2010, 2, 1–10. [Google Scholar]
- Liu, J.; Duan, Y. Saliva: A potential media for disease diagnostics and monitoring. Oral Oncol. 2012, 48, 569–577. [Google Scholar] [CrossRef]
- Rajkumar, K.; Nandhini, G.; Ramya, R.; Rajashree, P.; Kumar, A.R.; Anandan, S.N. Validation of the diagnostic utility of salivary interleukin 8 in the differentiation of potentially malignant oral lesions and oral squamous cell carcinoma in a region with high endemicity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Feeney, M.; Lazarus, H.; Nadler, L.M.; Colvin, R.B.; Knapp, R.C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J. Clin. Investig. 1981, 68, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Daoud, E.; Bodor, G. CA-125 concentrations in malignant and nonmalignant disease. Clin. Chem. 1991, 37, 1968–1974. [Google Scholar] [CrossRef]
- Sood, A.K.; Buller, R.E.; Burger, R.A.; Dawson, J.D.; Sorosky, J.I.; Berman, M. Value of preoperative CA 125 level in the management of uterine cancer and prediction of clinical outcome. Obs. Gynecol. 1997, 90, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13, 129–144. [Google Scholar] [CrossRef]
- Palmer, C.; Pratt, J.; Basu, B.; Earl, H. A study to evaluate the use of CA125 in ovarian cancer follow-up: A change in practice led by patient preference. Gynecol. Oncol. 2006, 101, 4–11. [Google Scholar] [CrossRef]
- Chen, D.X.; Schwartz, P.E.; Li, F.Q. Saliva and serum CA 125 assays for detecting malignant ovarian tumors. Obs. Gynecol. 1990, 75, 701–704. [Google Scholar]
- Zöller, J.; Fiehn, W.; Mende, U.; Hotz, G. The diagnostic value of the tumor markers CEA,” Ca 19-9”,” Ca 125”,” Ca15-3” and” SCC” for the detection of recurrent tumors in patients with tumors of the head and neck. Dtsch. Z. Mund Kiefer Gesichtschir. 1990, 14, 254–259. [Google Scholar] [PubMed]
- Hoffmann, J.; Munz, A.; Krimmel, M.; Alfter, G. Intraoperative and postoperative kinetics of serum tumor markers in patients with oral carcinoma. J. Oral Maxillofac. Surg. 1998, 56, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.F.; Du, M.; Han, J.X.; Zhang, M.; Tang, X.F.; Xing, R.D. Saliva CA125 and TPS levels in patients with oral squamous cell carcinoma. Int. J. Biol. Markers 2013, 28, 216–220. [Google Scholar] [CrossRef]
- Ahmad, A.; Ghaffar, U.B.; Hiware, S.D.; Ali, M.T.; Wasi, S. A prospective study for Assessment of saliva CA-125 level in oral cancer patients. Pak. J. Med. Health Sci. 2021, 15, 1922–1925. [Google Scholar] [CrossRef]
- Younus, S.; Younus, N.; Khan, H.M. Determination of CA 125 salivary level with clinicopathological characteristics in oral squamous cell carcinoma in Karachi. Asian J. Med. Health 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Osman, T.A.; Satti, A.A.; Boe, O.E.; Yang, Y.H.; Ibrahim, S.O.; Suleiman, A.M. Pattern of malignant tumors registered at a referral oral and maxillofacial hospital in Sudan during 2006 and 2007. J. Cancer Res. Ther. 2010, 6, 473–477. [Google Scholar] [CrossRef]
- Füzéry, A.K.; Levin, J.; Chan, M.M.; Chan, D.W. Translation of proteomic biomarkers into FDA approved cancer diagnostics: Issues and challenges. Clin. Proteom. 2013, 10, 13. [Google Scholar] [CrossRef]
- Rifai, N.; Gillette, M.A.; Carr, S.A. Protein biomarker discovery and validation: The long and uncertain path to clinical utility. Nat. Biotechnol. 2006, 24, 971–983. [Google Scholar] [CrossRef]
- Fernando, S.A.; Wilson, G.S. Studies of the ‘hook’effect in the one-step sandwich immunoassay. J. Immunol. Methods 1992, 151, 47–66. [Google Scholar] [CrossRef]
- McCauley, R.J.; Swisher, L. Psychometric review of language and articulation tests for preschool children. J. Speech Hear. Disord. 1984, 49, 34–42. [Google Scholar] [CrossRef]
- Zhou, X.H.; Obuchowski, N.A.; McClish, D.K. Statistical Methods in Diagnostic Medicine; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Chen, X.; Sandrine, I.K.; Yang, M.; Tu, J.; Yuan, X. MUC1 and MUC16: Critical for immune modulation in cancer therapeutics. Front. Immunol. 2024, 15, 1356913. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Q.; Xie, Y.; Li, M.; He, L.; Zhang, Q.; Yu, H.; Wang, Y.; Liu, J. MUC16 Stimulates Neutrophils to an Inflammatory and Immunosuppressive Phenotype in Ovarian Cancer. J. Ovarian Res. 2023, 16, 181. [Google Scholar] [CrossRef]
- Rajabi, H.; Hiraki, M.; Kufe, D. MUC1-C Activates Polycomb Repressive Complexes and Downregulates Tumor Suppressor Genes in Human Cancer Cells. Oncogene 2018, 37, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.A.J.; Vardhan, B.G.H.; Srinivasan, S.; Gopal, S.K. Evaluation of Salivary Interleukin-6 in Patients with Oral Squamous Cell Carcinoma, Oral Potentially Malignant Disorders, Chronic Periodontitis and in Healthy Controls—A Cross-Sectional Comparative Study. Ann. Maxillofac. Surg. 2023, 13, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Chen, J.; Yan, W.; Zang, D.; Qin, Q.; Deng, A.M. Diagnostic Accuracy of Cytokeratin-19 Fragment (CYFRA 21-1) for Bladder Cancer: A Systematic Review and Meta-Analysis. Tumour Biol. 2015, 36, 3137–3145. [Google Scholar] [CrossRef]
- Uenishi, T.; Kubo, S.; Hirohashi, K.; Tanaka, H.; Shuto, T.; Yamamoto, T.; Nishiguchi, S. Cytokeratin-19 Fragments in Serum (CYFRA 21-1) as a Marker in Primary Liver Cancer. Br. J. Cancer 2003, 88, 1894–1899. [Google Scholar] [CrossRef]



| Variable | OSCC Group (n = 50) | Control Group (n = 50) |
|---|---|---|
| Number of participants | 50 | 50 |
| Gender | ||
| Males | 34 (68%) | 34 (68%) |
| Females | 16 (32%) | 16 (32%) |
| Age | ||
| Mean age (years) | 59.72 ± 15.35 | 58.26 ± 14.98 |
| Toombak Use | ||
| Current dipper | 19 (38%) | 17 (34%) |
| Ex-dipper | 12 (24%) | 12 (24%) |
| Non-dipper | 19 (38%) | 21 (42%) |
| Alcohol Consumption | ||
| Current alcohol consumer | 3 (6%) | 3 (6%) |
| Ex-alcohol consumer | 10 (20%) | 10 (20%) |
| Non-alcohol consumer | 37 (74%) | 37 (74%) |
| Smoking | ||
| Current smoker | 12 (24%) | 12 (24%) |
| Ex-smoker | 11 (22%) | 11 (22%) |
| Non-smoker | 27 (54%) | 27 (54%) |
| Chronic Medical Condition | ||
| Presence of chronic medical condition | 9 (18%) | 8 (16%) |
| Medically fit | 41 (82%) | 42 (84%) |
| Stage | Number of Patients (n) | Percentage (%) |
|---|---|---|
| Stage I | 3 | 6% |
| Stage II | 3 | 6% |
| Stage III | 1 | 2% |
| Stage IV | 43 | 86% |
| Total | 50 | 100% |
| Histopathological Grade | N | Salivary CA-125 Level (U/mL) Median (Range) | Pairwise Comparison | Bonferroni-Corrected p-Value | Significance |
|---|---|---|---|---|---|
| Well differentiated (G1) | 34 | 336.50 (31.80–1000.00) | G1 vs. G2 | 1.000 | Not significant |
| Moderately differentiated (G2) | 11 | 278.10 (58.30–864.40) | G1 vs. G3 | 0.0117 | Significant |
| Poorly differentiated (G3) | 5 | 839.40 (472.40–1340.00) | G2 vs. G3 | 0.0261 | Significant |
| Site of OSCC | N | Level of Salivary CA-125 (Median and Range) U/mL |
|---|---|---|
| Multiple sites involved | 15 | 335.70 (31.80–1000.00) |
| Lips | 3 | 465.30 (96.60–740.00) |
| Anterior tongue | 1 | 151.80 |
| Floor of the mouth | 1 | 232.20 |
| Buccal mucosa | 6 | 520.30 (306.70–1340.00) |
| Alveolar ridge | 16 | 403.80 (45.20–956.00) |
| Hard palate | 4 | 410.20 (155.10–513.00) |
| Retromolar trigone | 2 | 238.30 (198.50–278.10) |
| Posterior tongue | 2 | 91.90 (58.30–125.50) |
| p-value | 0.326 | |
| Groups | Cases Group | Control Group | |||
|---|---|---|---|---|---|
| Gender | N | Level of Salivary CA-125 (Median and Range) U/mL | N | Level of Salivary CA-125 (Median and Range) U/mL | |
| Males | 34 | 324.20 (31.80–1000.00) | 34 | 188.60 (17.60–1000.00) | |
| Females | 16 | 484.90 (58.30–1340.00) | 16 | 248.15 (76.80–1011.20) | |
| p-value | 0.134 | 0.167 | |||
| Groups | Cases Group | Control Group | |||
|---|---|---|---|---|---|
| Toombak Use | N | Level of Salivary CA-125 (Median and Range) U/mL | N | Level of Salivary CA-125 (Median and Range) U/mL | |
| Non-Snuff Dipper | 19 | 355.60 (58.30–1340.00) | 21 | 227.80 (76.80–1011.20) | |
| Snuff Dipper | 19 | 205.70 (31.80–839.40) | 17 | 187.70 (37.00–1000.00) | |
| Ex-Snuff Dipper | 12 | 352.10 (125.50–1000.00) | 12 | 178.25 (17.60–633.90) | |
| p-value | 0.391 | 0.628 | |||
| Test Result | Group | Total | |
|---|---|---|---|
| Cases Group | Control Group | ||
| Positive | 24 | 11 | 35 |
| Negative | 26 | 39 | 65 |
| Total | 50 | 50 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, R.; El Sheikh, M.; Hashim, N.T.; Chaitanya, N.C.; Suleiman, A. Comparative Analysis of Salivary Tumor Marker CA-125 Among Oral Squamous Cell Carcinoma Patients and Healthy Individuals. Dent. J. 2025, 13, 194. https://doi.org/10.3390/dj13050194
Mohammed R, El Sheikh M, Hashim NT, Chaitanya NC, Suleiman A. Comparative Analysis of Salivary Tumor Marker CA-125 Among Oral Squamous Cell Carcinoma Patients and Healthy Individuals. Dentistry Journal. 2025; 13(5):194. https://doi.org/10.3390/dj13050194
Chicago/Turabian StyleMohammed, Riham, Mariam El Sheikh, Nada Tawfig Hashim, Nallan CSK Chaitanya, and Ahmed Suleiman. 2025. "Comparative Analysis of Salivary Tumor Marker CA-125 Among Oral Squamous Cell Carcinoma Patients and Healthy Individuals" Dentistry Journal 13, no. 5: 194. https://doi.org/10.3390/dj13050194
APA StyleMohammed, R., El Sheikh, M., Hashim, N. T., Chaitanya, N. C., & Suleiman, A. (2025). Comparative Analysis of Salivary Tumor Marker CA-125 Among Oral Squamous Cell Carcinoma Patients and Healthy Individuals. Dentistry Journal, 13(5), 194. https://doi.org/10.3390/dj13050194








