Quantification of Bisphenol A in the Saliva of Patients Wearing Clear Aligners
Abstract
1. Introduction
Hypothesis
2. Materials and Methods
2.1. Study Design, Participants, Sample Size and Ethical Considerations
- Patients beginning orthodontic treatment with clear aligners in the Master’s Program in Orthodontic Specialization at the University of Valencia.
- Patients with no existing composite restorations.
- Patients with no impairment of salivary secretion.
- Patients are not occupationally exposed to high levels of BPA.
- T0: Prior to the start of treatment without attachments or the aligner.
- T1: 30 min after bonding the attachments using the template with Transbond LR® composite (3M Unitek, Saint Paul, Minnesota, United States).
- T2: 30 min after placement of the aligners, that is, 60 min after bonding the attachments.
- T3: After one week of aligner use

- While seated in the dental chair, each patient was given a paraffin pellet to stimulate salivary secretion, enabling the collection of approximately 1.5–2 mL of saliva. Using a disposable pipette, the saliva was transferred into plastic centrifuge tubes with screw caps.
- The samples were individually placed in sterilization pouches, labeled with numerical codes, and stored in a freezer at −80 °C.
2.2. Sample Processing
- One milliliter of each thawed sample was collected and transferred into a new test tube.
- This milliliter of saliva was vortexed for 1 min.
- After vortexing, 4.11 µL of 37% hydrochloric acid and 2 mL of methyl tert-butyl ether were added to the sample, followed by an additional minute of vortex agitation. This step was performed to ensure proper sample processing and to precipitate proteins, thereby facilitating subsequent analysis by mass spectrometry.
- The mixture was then centrifuged at 3000 rpm for 5 min to separate the components.
- Then, 1 mL of the supernatant was collected into an Eppendorf tube and evaporated in an Eppendorf Concentrator 5301 at 60 °C for 10 or more minutes, depending on the sample, until the liquid was completely evaporated.
- The dry residues were reconstituted in 50 microliters of methanol and injected into a preparation plate for analysis using high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS) at the Jerónimo Muñoz research building laboratory on the Burjassot Campus of the University of Valencia.
2.3. Statistical Analysis
3. Results
Relevant Descriptive Data
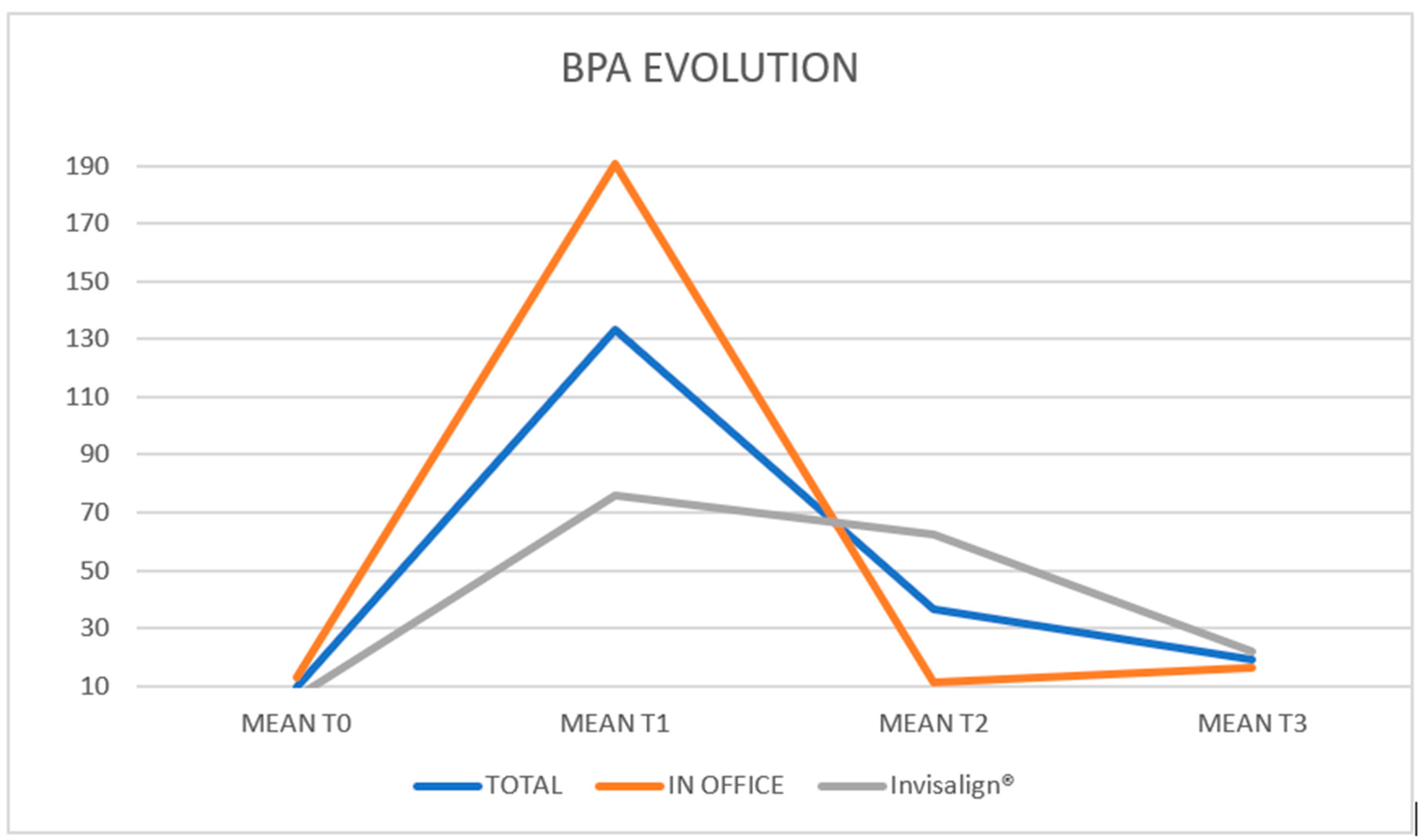
- Overall, BPA changes significantly during the follow-up period (p < 0.001). This variation should be considered similar in both groups (p = 0.545).
- Reciprocally, BPA measurements are significantly different between the two groups (p = 0.001); but the differences remain at all time points (p = 0.545).
| p-Value | |
|---|---|
| Time | <0.001 *** |
| Group | 0.001 ** |
| Time × Group | 0.545 |
- In the In Office group, the increase at T1 is followed by an immediate return to baseline levels, which are maintained until the weekly visit.
- In the Invisalign® group, the increase at T1 is followed by a more moderate decrease, with levels returning to a point that is not significantly different from the T1 peak or the baseline.
| In Office | Invisalign® | |
|---|---|---|
| T0 vs. T1 | 0.024 * | 0.048 * |
| T0 vs. T2 | 1 | 0.300 |
| T0 vs. T3 | 1 | 0.816 |
| T1 vs. T2 | 0.018 * | 1 |
| T1 vs. T3 | 0.012 * | 0.360 |
| T2 vs. T3 | 1 | 1 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPA | Bisphenol A |
References
- Howe, S.R.; Borodinsky, L. Potential exposure to bisphenol A from food-contact use of polycarbonate resins. Food Addit. Contam. 1998, 15, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, A.S.; Sathyanarayana, H.P.; Kailasam, V.; Padmanabhan, S. Comparative evaluation of salivary bisphenol A levels in patients wearing vacuum-formed and Hawley retainers: An in-vivo study. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 471–476. [Google Scholar] [CrossRef]
- Moreira, M.R.; Matos, L.G.; de Souza, I.D.; Brigante, T.A.; Queiroz, M.E.; Romano, F.L.; Nelson-Filho, P.; Matsumoto, M.A. Bisphenol A release from orthodontic adhesives measured in vitro and in vivo with gas chromatography. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 477–483. [Google Scholar] [CrossRef]
- Eliades, T. Fragen und Antworten zu Bisphenol A. IOK 2013, 45, 51–56. [Google Scholar] [CrossRef]
- Sifakakis, I.; Eliades, T. Adverse reactions to orthodontic materials. Aust. Dent. J. 2017, 62, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Görukmez, E.; Sen Yilmaz, B.; Ramoglu, S.I. Is a Single Rinse Effective on Evacuating the Residual Monomers After Orthodontic Bonding? An In Vivo Study. Bezmialem Sci. 2021, 9, 127–133. [Google Scholar] [CrossRef]
- Iliadi, A.; Koletsi, D.; Papageorgiou, S.N.; Eliades, T. Safety Considerations for Thermoplastic-Type Appliances Used as Orthodontic Aligners or Retainers. A Systematic Review and Meta-Analysis of Clinical and In-Vitro Research. Materials 2020, 13, 1843. [Google Scholar] [CrossRef]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef] [PubMed]
- Prokop, Z.; Hanková, L.; Jeřábek, K. Bisphenol A synthesis—Modeling of industrial reactor and catalyst deactivation. React. Funct. Polym. 2004, 60, 77–83. [Google Scholar] [CrossRef]
- Eliades, T.; Pratsinis, H.; Athanasiou, A.E.; Eliades, G.; Kletsas, D. Cytotoxicity and estrogenicity of Invisalign appliances. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 100–103. [Google Scholar] [CrossRef]
- Ziuchkovski, J.P.; Fields, H.W.; Johnston, W.M.; Lindsey, D.T. Assessment of perceived orthodontic appliance attractiveness. Am. J. Orthod. Dentofac. Orthop. 2008, 133, S68–S78. [Google Scholar] [CrossRef]
- Walton, D.K.; Fields, H.W.; Johnston, W.M.; Rosenstiel, S.F.; Firestone, A.R.; Christensen, J.C. Orthodontic appliance preferences of children and adolescents. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 698.e1–698.e12. [Google Scholar] [CrossRef] [PubMed]
- Ireland, A.J.; Hosein, I.; Sherriff, M. Enamel loss at bond-up, debond and clean-up following the use of a conventional light-cured composite and a resin-modified glass polyalkenoate cement. Eur. J. Orthod. 2005, 27, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, A.; Papageorgiou, S.N.; Sifakakis, I.; Zinelis, S.; Eliades, G.; Eliades, T. Orthodontic bonding and debonding induces structural changes but does not alter the mechanical properties of enamel. Prog. Orthod. 2018, 19, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eliades, T. Bisphenol A and orthodontics: An update of evidence-based measures to minimize exposure for the orthodontic team and patients. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 435–441, Erratum in Am. J. Orthod. Dentofac. Orthop. 2017, 152, 740.. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. for the STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational, studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.G.; Kim, J.Y.; Kim, J.; Won, P.J.; Nam, J.H. Release of bisphenol A from resin composite used to bond orthodontic lingual retainers. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Vítores-Calero, A.; Zamora-Martínez, N.; Bellot-Arcís, C.; Tarazona-Álvarez, B.; Montiel-Company, J.M.; García-Sanz, V.; Paredes-Gallardo, V. Biological significance of long-term bisphenol A release in the saliva of patients wearing orthodontic appliances: A systematic review and meta-analysis. J. Clin. Exp. Dent. 2024, 16, e912–e920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stocker, L.; Zervou, S.K.; Papageorgiou, S.N.; Karakousoglou, S.; Triantis, T.; Hiskia, A.; Eliades, G.; Eliades, T. Salivary levels of eluents during Invisalign™ treatment with attachments: An in vivo investigation. Prog. Orthod. 2024, 25, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manoj, M.K.; Ramakrishnan, R.; Babjee, S.; Nasim, R. High-performance liquid chromatography analysis of salivary bisphenol A levels from light-cured and chemically cured orthodontic adhesives. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M. Degradation and formation of bisphenol A in polycarbonate used in dentistry. J. Med. Dent. Sci. 2004, 51, 1–6. [Google Scholar]
- Kloukos, D.; Sifakakis, I.; Voutsa, D.; Doulis, I.; Eliades, G.; Katsaros, C.; Eliades, T. BPA qualtitative and quantitative assessment associated with orthodontic bonding in vivo. Dent. Mater. 2015, 31, 887–894. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Re-evaluation of the risks to public health related to the presence of bisphenol A(BPA) in foodstuffs. EFSA J. 2023, 21, e06857. [Google Scholar] [CrossRef]
- Bagley, B.D.; Smith, J.N.; Teeguarden, J.G. Risk assessment of predicted serum concentrations of bisphenol A in children and adults following treatment with dental composite restoratives, dental sealants, or orthodontic adhesives using physiologically based pharmacokinetic modeling. Regul. Toxicol. Pharmacol. 2021, 120, 104839. [Google Scholar] [CrossRef] [PubMed]
| Average BPA Count Values | Effect Size (d) | Power Achieved | ||
|---|---|---|---|---|
| 70% | 80% | 90% | ||
| 0.46–1.50 | 0.2 (small) | 151 | 192 | 256 |
| 0.46–3.04 | 0.5 (medium) | 27 | 33 | 44 |
| 0.46–5.04 | 0.89 (large) | 10 | 12 | 16 |
| N | % | |
|---|---|---|
| Total | 24 | 100.0% |
| In Office | 12 | 50.0% |
| Invisalign® | 12 | 50.0% |
| Global | |
|---|---|
| T0 vs. T1 | <0.001 *** |
| T0 vs. T2 | 0.456 |
| T0 vs. T3 | 0.660 |
| T1 vs. T2 | 0.024 * |
| T1 vs. T3 | <0.001 *** |
| T2 vs. T3 | 1 |
| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
| Group | 0.048 * | 0.640 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitores-Calero, A.; García-Sanz, V.; Paredes-Gallardo, V.; Zamora-Martínez, N.; Tarazona-Álvarez, B. Quantification of Bisphenol A in the Saliva of Patients Wearing Clear Aligners. Dent. J. 2025, 13, 599. https://doi.org/10.3390/dj13120599
Vitores-Calero A, García-Sanz V, Paredes-Gallardo V, Zamora-Martínez N, Tarazona-Álvarez B. Quantification of Bisphenol A in the Saliva of Patients Wearing Clear Aligners. Dentistry Journal. 2025; 13(12):599. https://doi.org/10.3390/dj13120599
Chicago/Turabian StyleVitores-Calero, Andrea, Verónica García-Sanz, Vanessa Paredes-Gallardo, Natalia Zamora-Martínez, and Beatriz Tarazona-Álvarez. 2025. "Quantification of Bisphenol A in the Saliva of Patients Wearing Clear Aligners" Dentistry Journal 13, no. 12: 599. https://doi.org/10.3390/dj13120599
APA StyleVitores-Calero, A., García-Sanz, V., Paredes-Gallardo, V., Zamora-Martínez, N., & Tarazona-Álvarez, B. (2025). Quantification of Bisphenol A in the Saliva of Patients Wearing Clear Aligners. Dentistry Journal, 13(12), 599. https://doi.org/10.3390/dj13120599









