Insights into the Cytotoxicity and Irritant Potential of Chlorhexidine Digluconate: An In Vitro and In Ovo Safety Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Computational Assessment of CHX in Terms of Drug Likeness and Potential Toxicity
2.3. Cell Culture Conditions
2.4. Applied Treatment Regimen
2.5. Cell Viability Assay
2.6. Assessment of Cell Confluence and Cell Number
2.7. Evaluation of Lactate Dehydrogenase (LDH) Leakage
2.8. Bright-Field Cell Morphology Assessment
2.9. TexasRed™-X Phalloidin Staining
2.10. Hoechst 33342 Nuclear Staining
2.11. The HET-CAM Assay
3. Results
3.1. Computational Predictions of CHX Drug Likeness and Toxic Risk
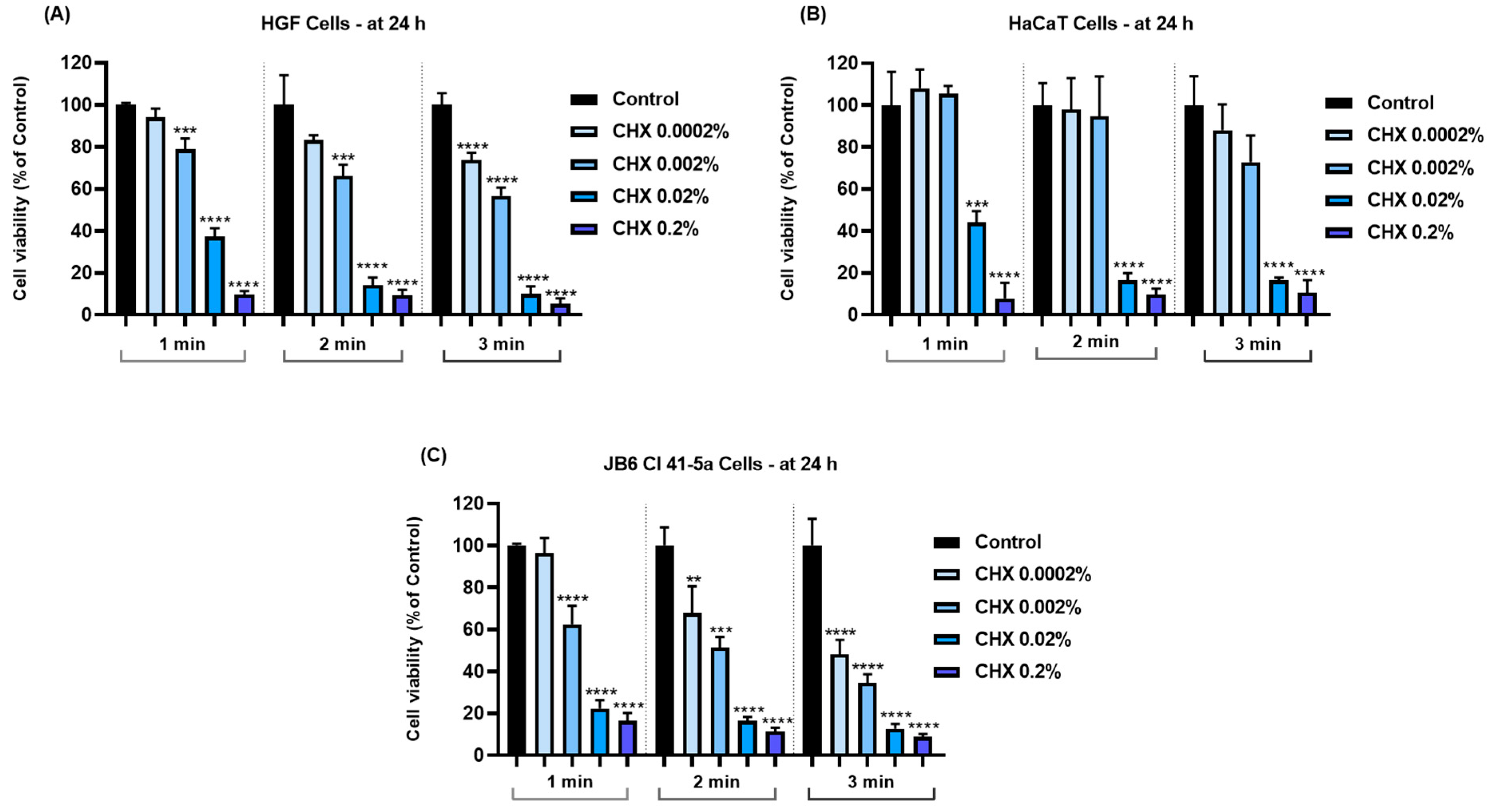
3.2. CHX Impairs Cell Viability in a Concentration-Dependent Manner
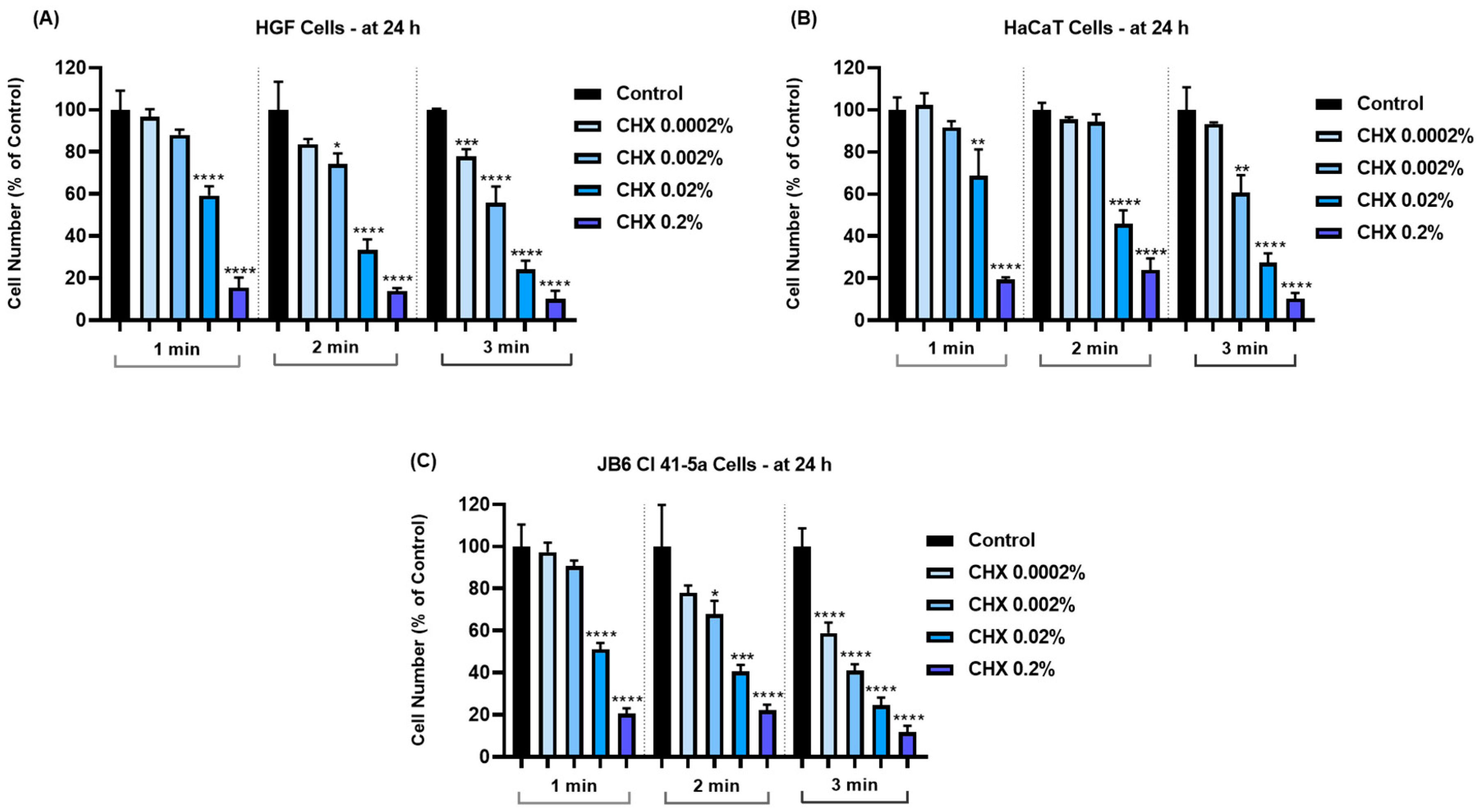
3.3. CHX Reduces Cell Number and Confluence in a Concentration-Dependent Manner
3.4. CHX-Induced Cell Membrane Damage and Lactate Dehydrogenase Leakage
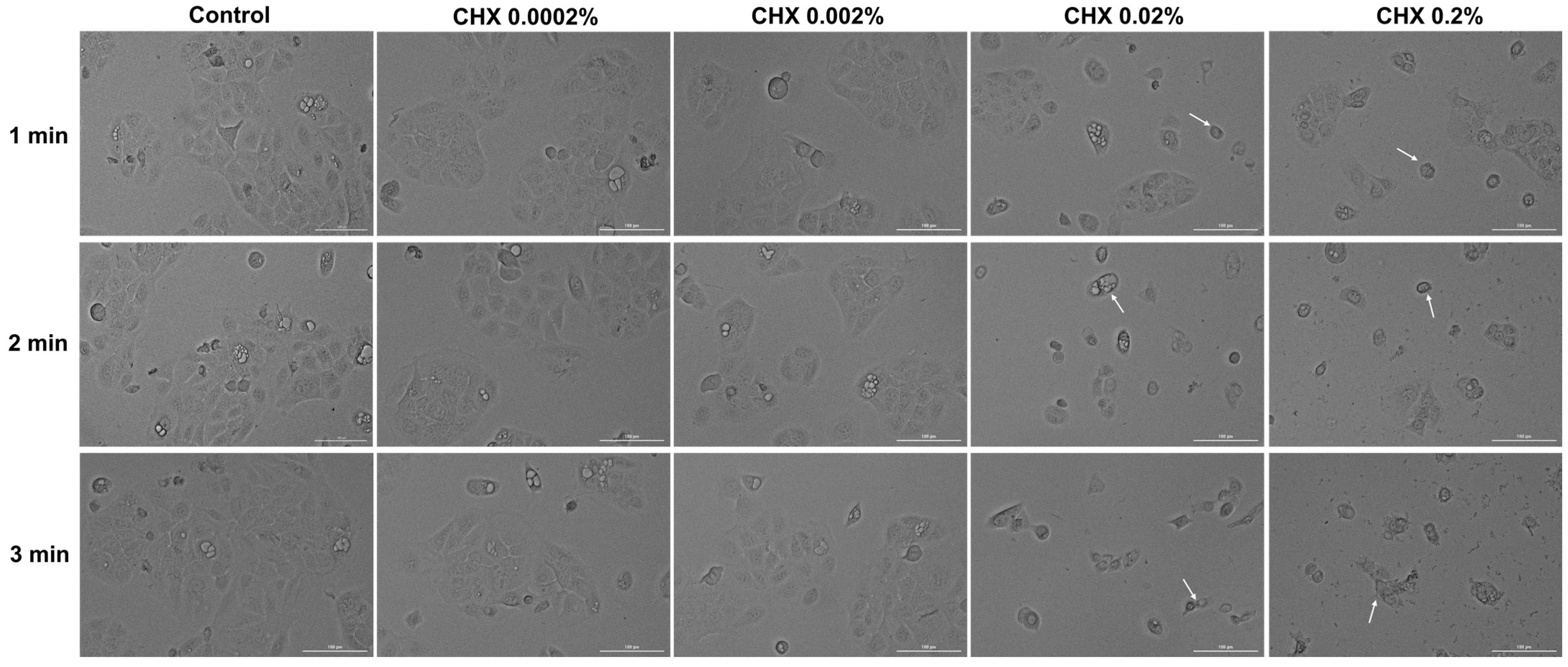
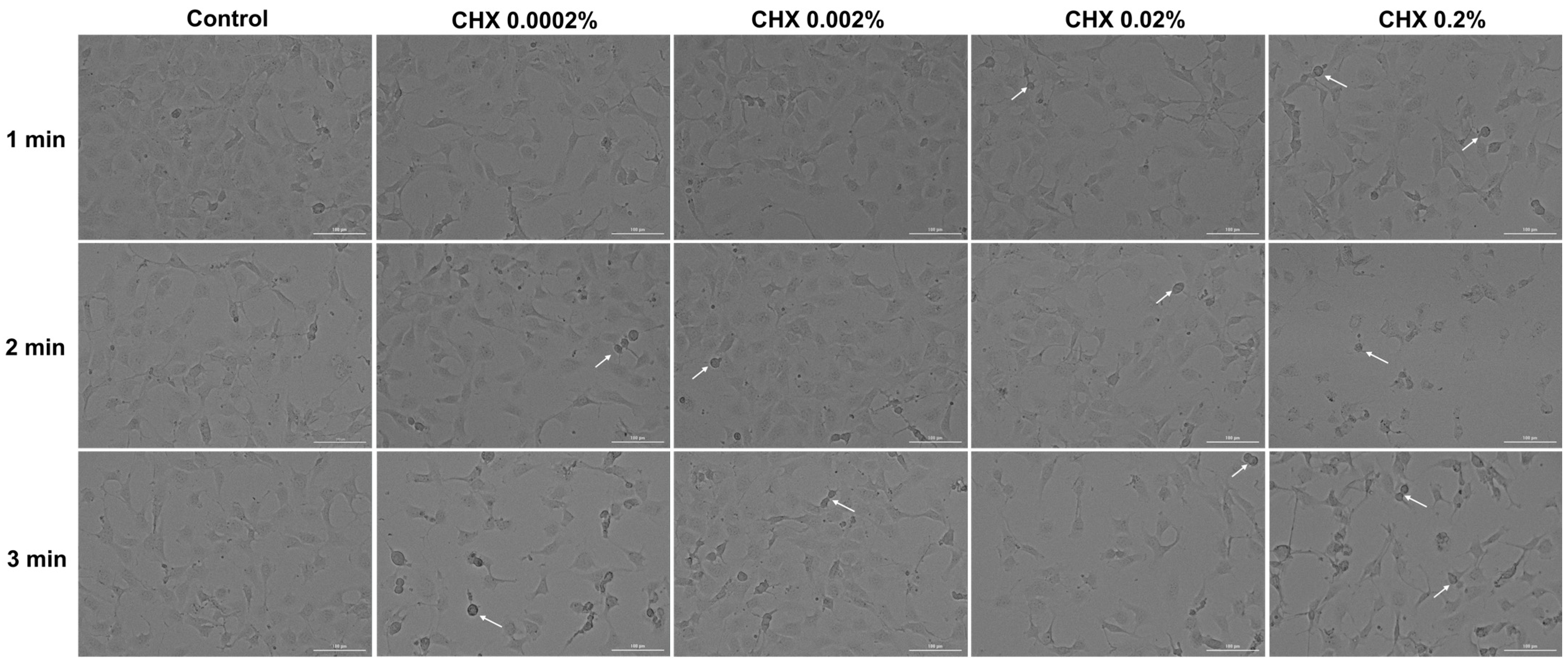
3.5. CHX-Induced Changes in Cell Morphology
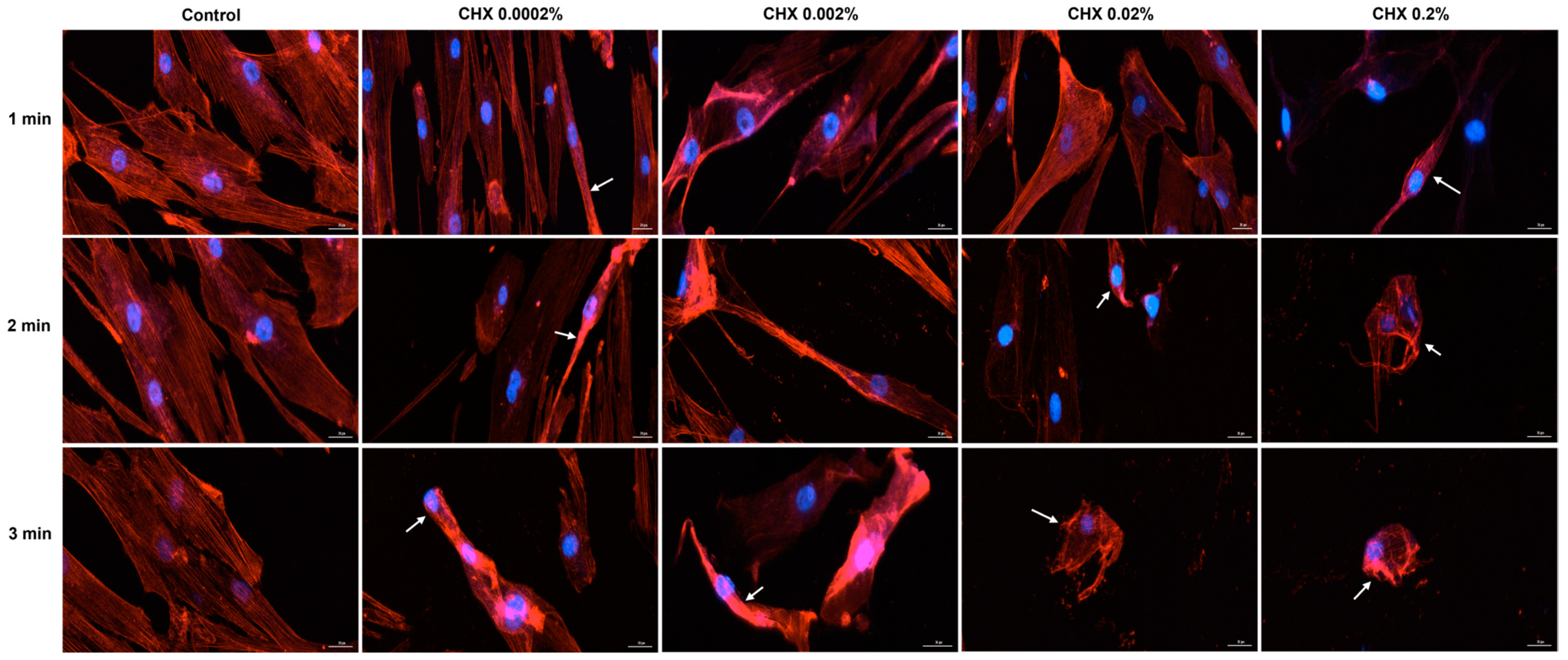
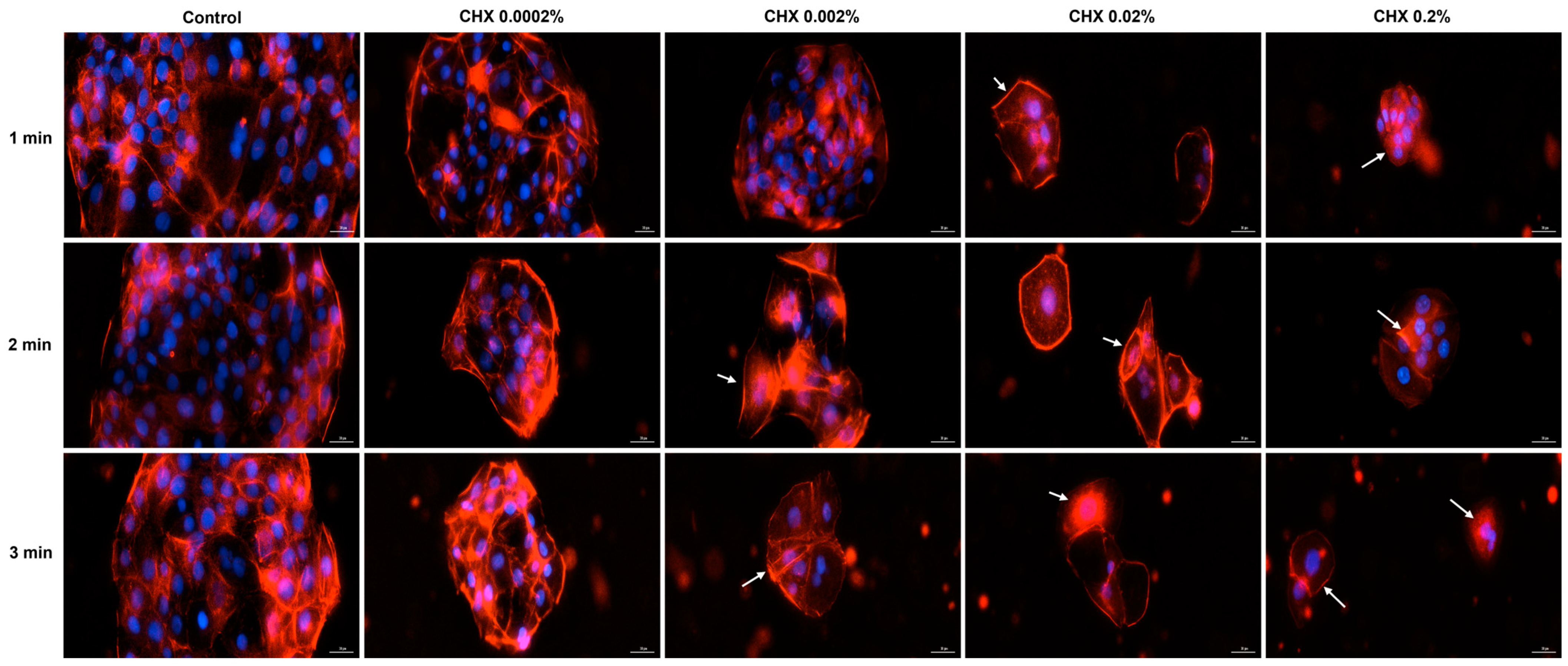
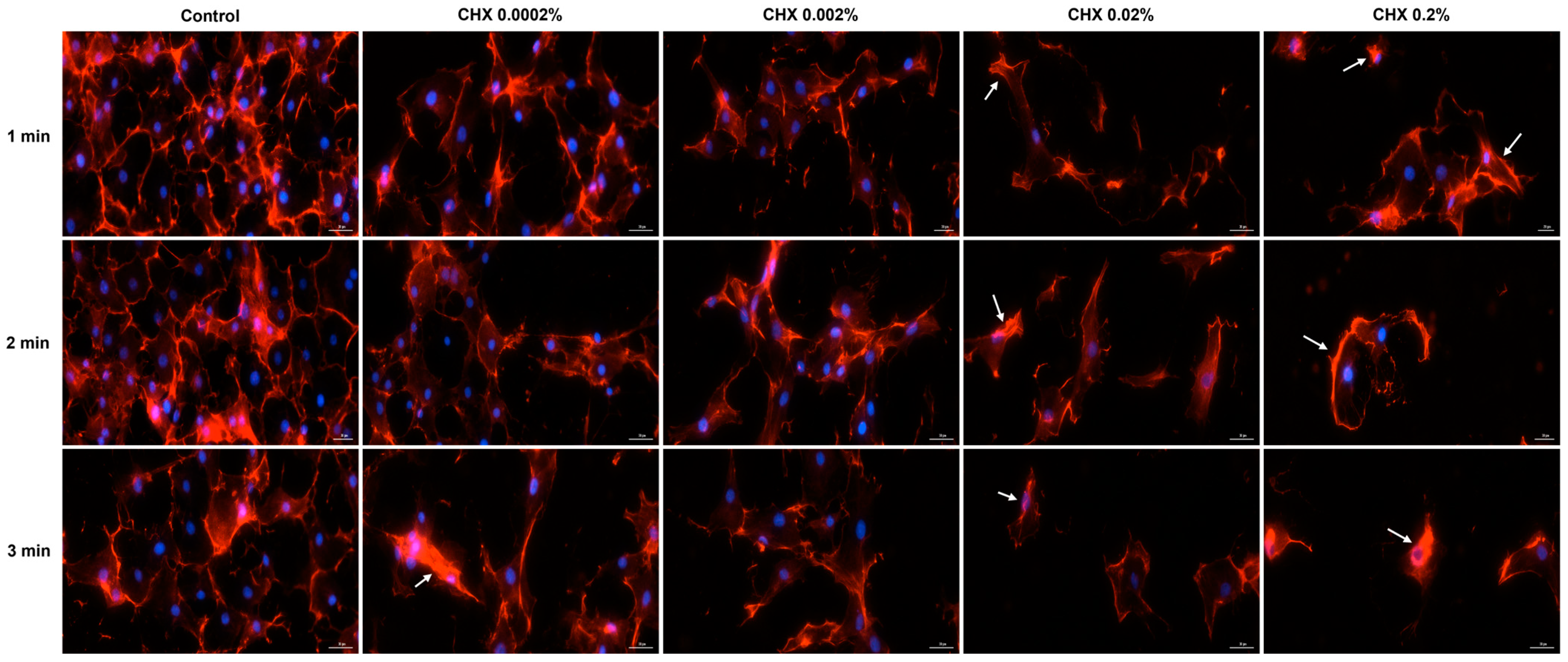
3.6. CHX-Induced F-Actin Rearrangements
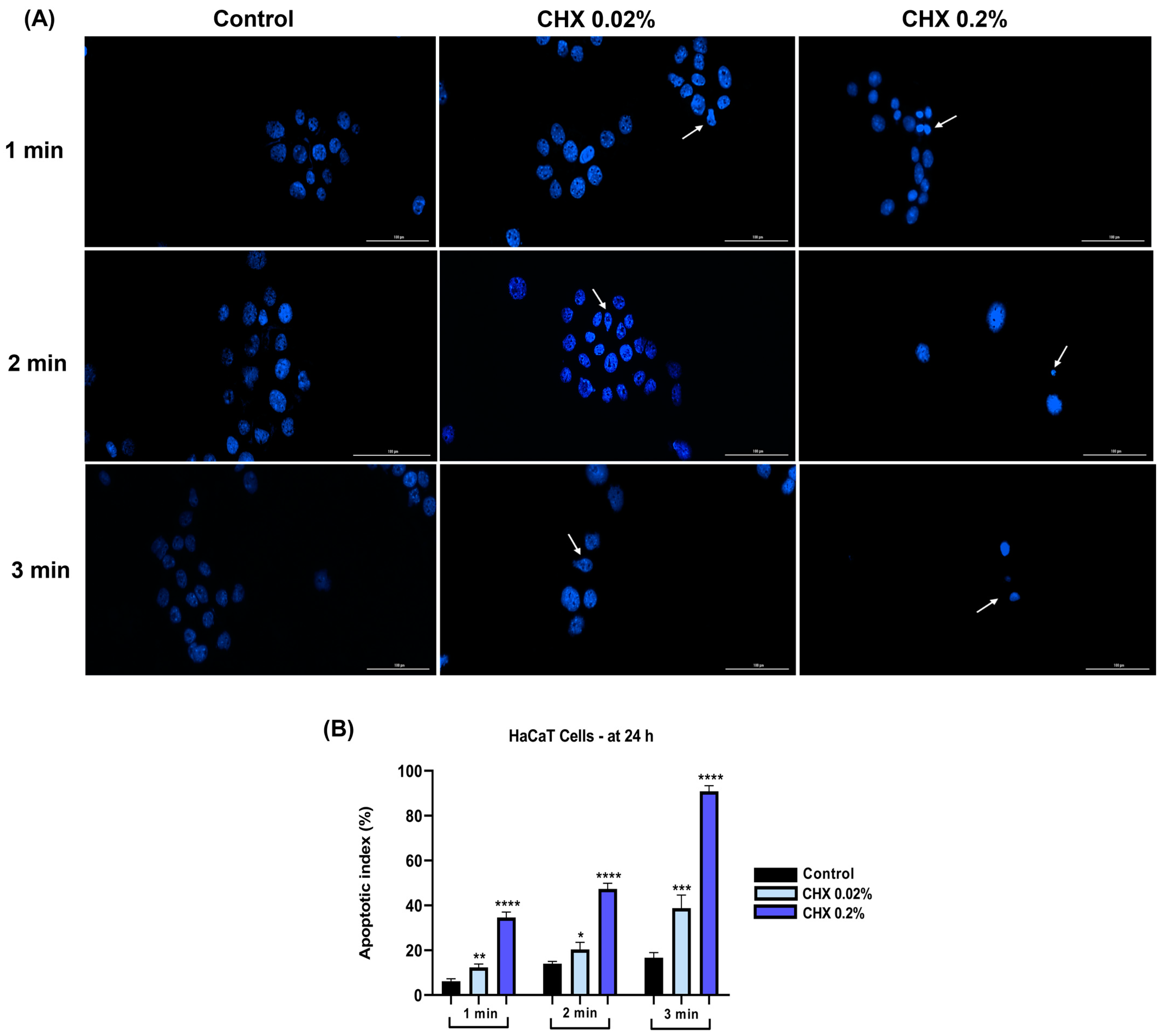
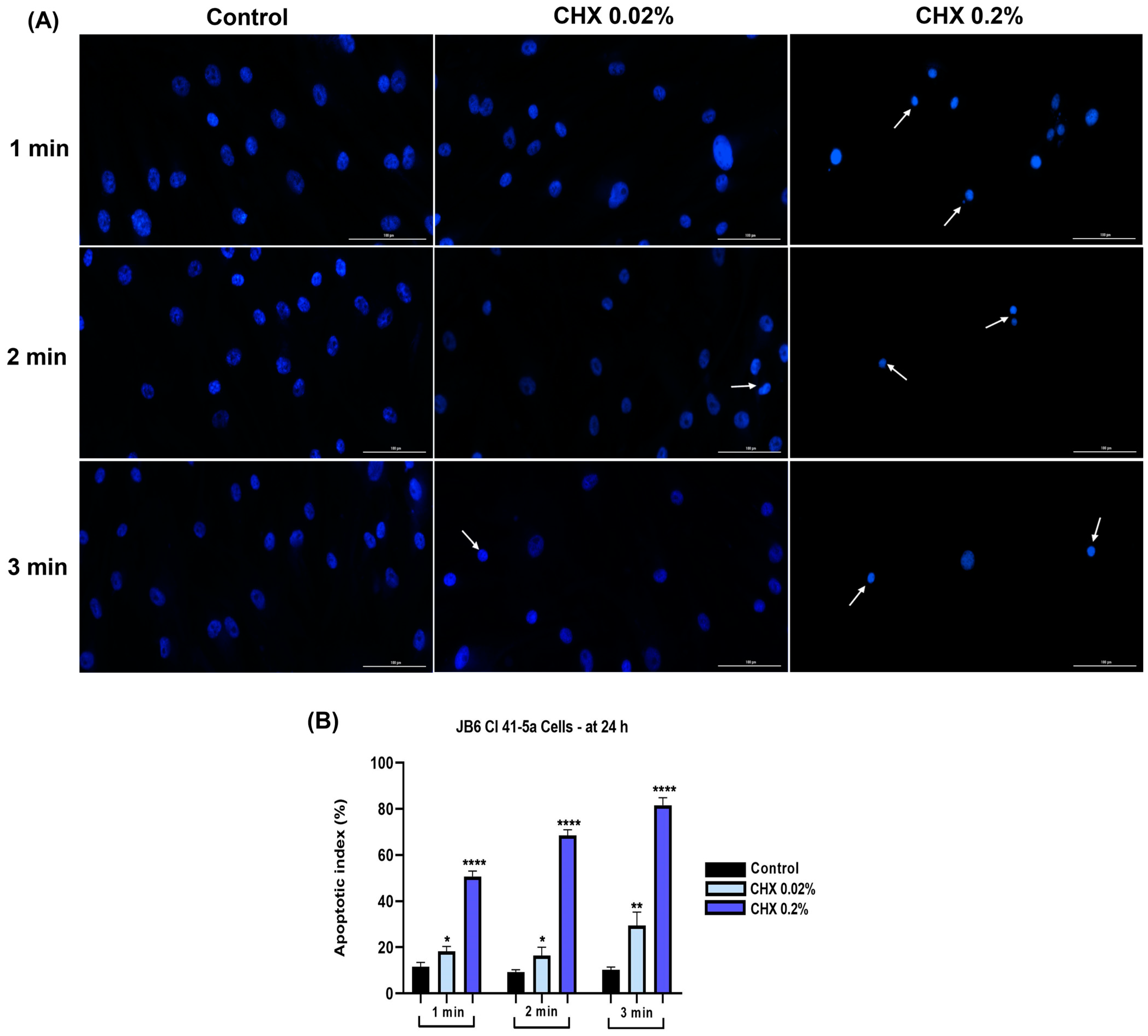
3.7. CHX 0.02% and 0.2% Induced an Apoptotic-like Nuclear Morphology
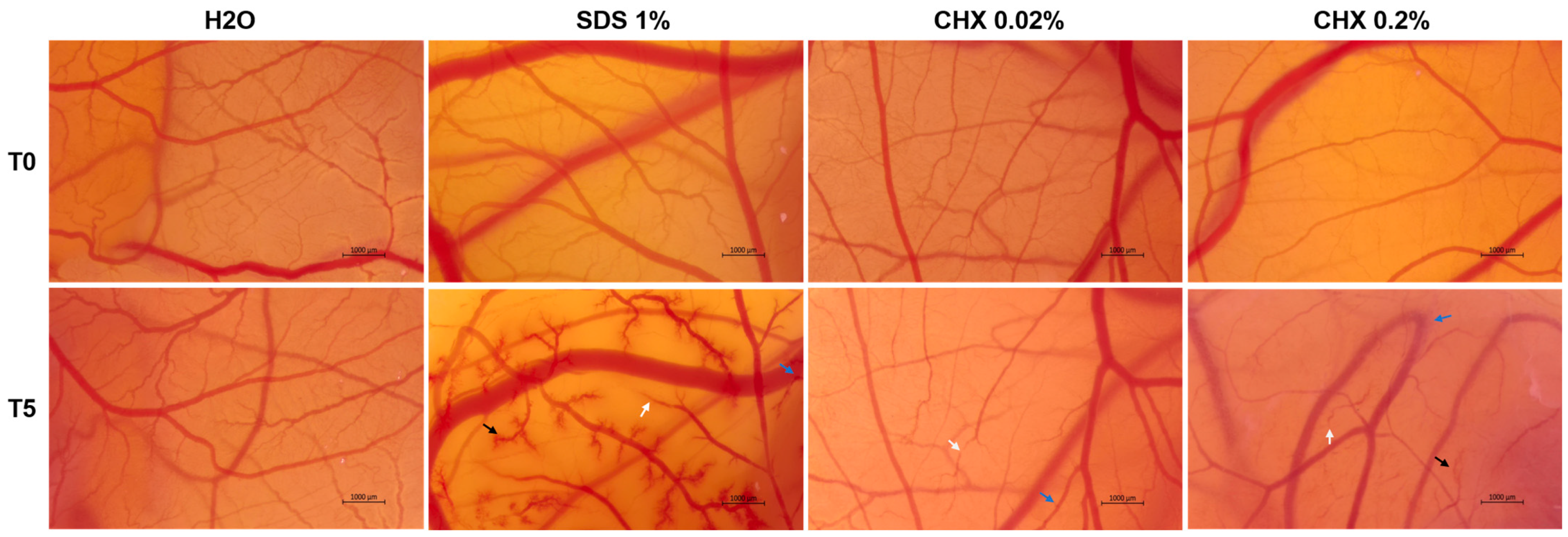
3.8. CHX 0.02% and 0.2% Exerted an Irritant Effect on the Chorioallantoic Membrane
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, K.S.; Kam, P.C. Chlorhexidine--pharmacology and clinical applications. Anaesth. Intensive Care 2008, 36, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, Z.; Nowakowska-Toporowska, A.; Weżgowiec, J.; Nowakowska, D. Design and characteristics of new experimental chlorhexidine dental gels with anti-staining properties. Adv. Clin. Exp. Med. 2019, 28, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Pałka, Ł.; Nowakowska-Toporowska, A.; Dalewski, B. Is Chlorhexidine in Dentistry an Ally or a Foe? A Narrative Review. Healthcare 2022, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.; Tarce, M.; Lodi, G.; Carrassi, A. Chlorhexidine (CHX) in dentistry: State of the art. Minerva Stomatol. 2012, 61, 399–419. [Google Scholar] [PubMed]
- Chiewchalermsri, C.; Sompornrattanaphan, M.; Wongsa, C.; Thongngarm, T. Chlorhexidine Allergy: Current Challenges and Future Prospects. J. Asthma Allergy 2020, 13, 127–133. [Google Scholar] [CrossRef] [PubMed]
- About Chlorhexidine: Mechanism of Action. Available online: https://www.chlorhexidinefacts.com/mechanism-of-action.html (accessed on 21 March 2024).
- Balloni, S.; Locci, P.; Lumare, A.; Marinucci, L. Cytotoxicity of three commercial mouthrinses on extracellular matrix metabolism and human gingival cell behaviour. Toxicol. Vitr. 2016, 34, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Łukomska-Szymańska, M.; Sokołowski, J.; Łapińska, B. Chlorhexidine—Mechanism of action and its application to dentistry. J. Stoma. 2017, 70, 405–417. [Google Scholar]
- Reda, B.; Hollemeyer, K.; Trautmann, S.; Volmer, D.A.; Hannig, M. First insights into chlorhexidine retention in the oral cavity after application of different regimens. Clin. Oral Investig. 2021, 25, 6109–6118. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.; Clements, M.N.; Azmery, K.S.; Bekker, A.; Bielicki, J.; Dramowski, A. Safety and efficacy of whole-body chlorhexidine gluconate cleansing with or without emollient in hospitalised neonates (NeoCHG): A multicentre, randomised, open-label, factorial pilot trial. EClinicalMedicine 2024, 69, 102463. [Google Scholar] [CrossRef] [PubMed]
- Lessa, F.C.R.; Aranha, A.M.F.; Nogueira, I.; Giro, E.M.A.; Hebling, J.; de Souza Costa, C.A. Toxicity of chlorhexidine on odontoblast-like cells. J. Appl. Oral Sci. 2010, 18, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Verma, U.P.; Gupta, A.; Yadav, R.K.; Tiwari, R.; Sharma, R.; Balapure, A.K. Cytotoxicity of chlorhexidine and neem extract on cultured human gingival fibroblasts through fluorescence-activated cell sorting analysis: An in-vitro study. Eur. J. Dent. 2018, 12, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kuan, Y.H.; Lee, T.H.; Huang, F.M.; Chang, Y.C. Assessment of the cytotoxicity of chlorhexidine by employing an in vitro mammalian test system. J. Dent. Sci. 2014, 9, 130–135. [Google Scholar] [CrossRef]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; D’Amico, C.; Mehta, V.; Cicciù, M.; Cervino, G. Chlorhexidine cytotoxicity on oral Behaviors: Last 20 Years systematic review. Oral Oncol. Rep. 2024, 9, 100245. [Google Scholar] [CrossRef]
- Reda, B.; Hollemeyer, K.; Trautmann, S.; Hannig, M.; Volmer, D.A. Determination of chlorhexidine retention in different oral sites using matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Arch. Oral Biol. 2020, 110, 104623. [Google Scholar] [CrossRef] [PubMed]
- Beckerman, S.; Lee, D.Y. Clinical implications of the erroneous use of the Verhoeff Stereopter: A case report. J. Am. Optom. Assoc. 1994, 65, 328–331. [Google Scholar] [PubMed]
- Dahl, J.E. Potential of dental adhesives to induce mucosal irritation evaluated by the HET–CAM method. Acta Odontol. Scand. 2007, 65, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.E. Irritation of dental adhesive agents evaluated by the HET–CAM test. Toxicol. Vitr. 1999, 13, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Coricovac, D.; Pinzaru, I.; Marcovici, I.; Macasoi, I.G.; Semenescu, A.; Lazar, G.; Cinta Pinzaru, S.; Radulov, I.; Alexa, E.; et al. Rutin bioconjugates as potential nutraceutical prodrugs: An in vitro and in ovo toxicological screening. Front. Pharmacol. 2022, 13, 1000608. [Google Scholar] [CrossRef]
- Chlorhexidine. Available online: https://pubchem.ncbi.nlm.nih.gov/com (accessed on 27 May 2024).
- Agilent. Available online: https://www.agilent.com/ (accessed on 27 May 2024).
- Wu, J.; Chen, Z.; Liu, Q.; Zeng, W.; Wu, X.; Lin, B. Silencing of Kv1. 5 Gene Inhibits Proliferation and Induces Apoptosis of Osteosarcoma Cells. Int. J. Mol. Sci. 2015, 16, 26914–26926. [Google Scholar] [PubMed]
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab-Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef] [PubMed]
- Budai, P.; Kormos, É.; Buda, I.; Somody, G.; Lehel, J. Comparative evaluation of HET-CAM and ICE methods for objective assessment of ocular irritation caused by selected pesticide products. Toxicol Vitr. 2021, 74, 105150. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef] [PubMed]
- Chavarría-Bolaños, D.; Esparza-Villalpando, V.; Ramírez, K. Antibacterial and Antifungal Capacity of Three Commercially Available Mouthwashes with Different Concentrations of Chlorhexidine. Odovtos Int. J. Dent. Sci. 2022, 24, 57–68. [Google Scholar] [CrossRef]
- Babiker, A.; Lutgring, J.D.; Fridkin, S.; Hayden, M.K. Assessing the Potential for Unintended Microbial Consequences of Routine Chlorhexidine Bathing for Prevention of Healthcare-associated Infections. Clin. Infect. Dis. 2021, 72, 891–898. [Google Scholar] [CrossRef]
- Khan, T.; Ahmad, R.; Azad, I.; Raza, S.; Joshi, S.; Khan, A.R. Computer-aided drug design and virtual screening of targeted combinatorial libraries of mixed-ligand transition metal complexes of 2-butanone thiosemicarbazone. Comput. Biol. Chem. 2018, 75, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Wielento, A.; Lagosz-Cwik, K.B.; Potempa, J.; Grabiec, A.M. The Role of Gingival Fibroblasts in the Pathogenesis of Periodontitis. J. Dent. Res. 2023, 102, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Komine, M.; Meijuan, J.; Kimura-Sashikawa, M.; Hossain, R.M.D.; Ansary, T.M.; Oshio, T.; Meephansan, J.; Tsuda, H.; Tominaga, S.-I.; Ohtsuki, M. Keratinocytes in Skin Disorders: The Importance of Keratinocytes as a Barrier. Keratinocyte Biology—Structure and Function in the Epidermis; IntechOpen: London, UK, 2022. [Google Scholar]
- Tabatabaei, M.H.; Mahounak, F.S.; Asgari, N.; Moradi, Z. Cytotoxicity of the Ingredients of Commonly Used Toothpastes and Mouthwashes on Human Gingival Fibroblasts. Front. Dent. 2019, 16, 450–457. [Google Scholar]
- Saweres-Argüelles, C.; Ramírez-Novillo, I.; Vergara-Barberán, M.; Carrasco-Correa, E.J.; Lerma-García, M.J.; Simó-Alfonso, E.F. Skin absorption of inorganic nanoparticles and their toxicity: A review. Eur. J. Pharm. Biopharm. 2023, 182, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Tewari-Singh, N.; Gu, M.; Agarwal, C.; White, C.W.; Agarwal, R. Biological and molecular mechanisms of sulfur mustard analogue-induced toxicity in JB6 and HaCaT cells: Possible role of ataxia telangiectasia-mutated/ataxia telangiectasia-Rad3-related cell cycle checkpoint pathway. Chem. Res. Toxicol. 2010, 23, 1034–1044. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay; Cold Spring Harb Protocotols: New York, NY, USA, 2018. [Google Scholar]
- Vörös, P.; Dobrindt, O.; Perka, C.; Windisch, C.; Matziolis, G.; Röhner, E. Human osteoblast damage after antiseptic treatment. Int. Orthop. 2014, 38, 177–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, M.; Jin, J.; Liu, Y.; Ben, C.; Li, H.; Cheng, D.; Sun, Y.; Guang-Yi, W.; Zhu, S. Analysis of povidone iodine, chlorhexidine acetate and polyhexamethylene biguanide as wound disinfectants: In vitro cytotoxicity and antibacterial activity. BMJ Nutr. Prev. Health 2023, 6, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Doonan, F.; Cotter, T.G. Morphological assessment of apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhao, W.; Cao, L.; Huang, J. Involvement of the Actin Machinery in Programmed Cell Death. Front. Cell Dev. Biol. 2021, 8, 634849. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. Structure and Organization of Actin Filaments. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, UK, 2000. [Google Scholar]
- Kubitschke, H.; Schnauss, J.; Nnetu, K.D.; Warmt, E.; Stange, R.; Kaes, J. Actin and Microtubule Networks Contribute Differently to Cell Response for Small and Large Strains. New J. Phys. 2017, 19, 093003. [Google Scholar] [CrossRef]
- Lian, N.; Wang, X.; Jing, Y.; Lin, J. Regulation of Cytoskeleton-associated Protein Activities: Linking Cellular Signals to Plant Cytoskeletal Function. J. Integr. Plant Biol. 2021, 63, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-B.; Lee, G.; Yun, B.G.; Kim, C.-H.; Ko, Y. Comparative Effects of Chlorhexidine and Essential Oils Containing Mouth Rinse on Stem Cells Cultured on a Titanium Surface. Mol. Med. Rep. 2014, 9, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Chellini, F.; Margheri, M.; Tonelli, P.; Tani, A. Effect of chlorhexidine digluconate on different cell types: A molecular and ultrastructural investigation. Toxicol. Vitr. 2008, 22, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Jorge Murillo, G.; Pérez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Harnoss, J.C.; Elrub, Q.M.A.; Jung, J.O.; Koburger, T.; Assadian, O.; Dissemond, J.; Baguhl, R.; Papke, R.; Kramer, A.; Supported by the Working Group Antiseptics of the International Society of Chemotherapy for Infection and Cancer (ISC). Irritative potency of selected wound antiseptics in the hen’s egg test on chorioallantoic membrane to predict their compatibility to wounds. Wound Repair Regen. 2019, 27, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jain, A.; Singla, M. Is saltwater mouth rinse as effective as chlorhexidine following periodontal surgery? Evid. Based Dent. 2021, 22, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Klausner, M.; Handa, Y.; Aizawa, S. In vitro three-dimensional organotypic culture models of the oral mucosa. Vitr. Cell Dev. Biol. Anim. 2021, 57, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Kandarova, H.; Hayden, P.J. Standardised Reconstructed Skin Models in Toxicology and Pharmacology: State of the Art and Future Development. Handb. Exp. Pharmacol. 2020, 265, 57–71. [Google Scholar]




| CHX | MW | cLogP | Solubility | Drug-Likeness | Drug Score | Mutagenic, Tumorigenic, Irritant, and Reproductive Toxic Potential |
|---|---|---|---|---|---|---|
| 504.0 | 3.72 | −7.92 | −2.988 | 0.182 | No risk |
| Sample | IS | Irritation Category |
|---|---|---|
| H2O | 0.07 | Non-irritant |
| SDS 1% | 19.68 | Severely Irritant |
| CHX 0.02% | 8.55 | Irritant |
| CHX 0.2% | 11.96 | Severely Irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, S.; Matichescu, A.; Buzatu, R.; Marcovici, I.; Geamantan-Sirbu, A.; Semenescu, A.D.; Bratu, R.C.; Bratu, D.-C. Insights into the Cytotoxicity and Irritant Potential of Chlorhexidine Digluconate: An In Vitro and In Ovo Safety Screening. Dent. J. 2024, 12, 221. https://doi.org/10.3390/dj12070221
Dinu S, Matichescu A, Buzatu R, Marcovici I, Geamantan-Sirbu A, Semenescu AD, Bratu RC, Bratu D-C. Insights into the Cytotoxicity and Irritant Potential of Chlorhexidine Digluconate: An In Vitro and In Ovo Safety Screening. Dentistry Journal. 2024; 12(7):221. https://doi.org/10.3390/dj12070221
Chicago/Turabian StyleDinu, Stefania, Anamaria Matichescu, Roxana Buzatu, Iasmina Marcovici, Andreea Geamantan-Sirbu, Alexandra Denisa Semenescu, Remus Christian Bratu, and Dana-Cristina Bratu. 2024. "Insights into the Cytotoxicity and Irritant Potential of Chlorhexidine Digluconate: An In Vitro and In Ovo Safety Screening" Dentistry Journal 12, no. 7: 221. https://doi.org/10.3390/dj12070221
APA StyleDinu, S., Matichescu, A., Buzatu, R., Marcovici, I., Geamantan-Sirbu, A., Semenescu, A. D., Bratu, R. C., & Bratu, D.-C. (2024). Insights into the Cytotoxicity and Irritant Potential of Chlorhexidine Digluconate: An In Vitro and In Ovo Safety Screening. Dentistry Journal, 12(7), 221. https://doi.org/10.3390/dj12070221









