Biological Effects of Orthodontic Tooth Movement on the Periodontium in Regenerated Bone Defects: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Information Sources and Search Strategy
2.3. Study Selection and Eligibility Criteria
2.4. Data Extraction and Synthesis
3. Results
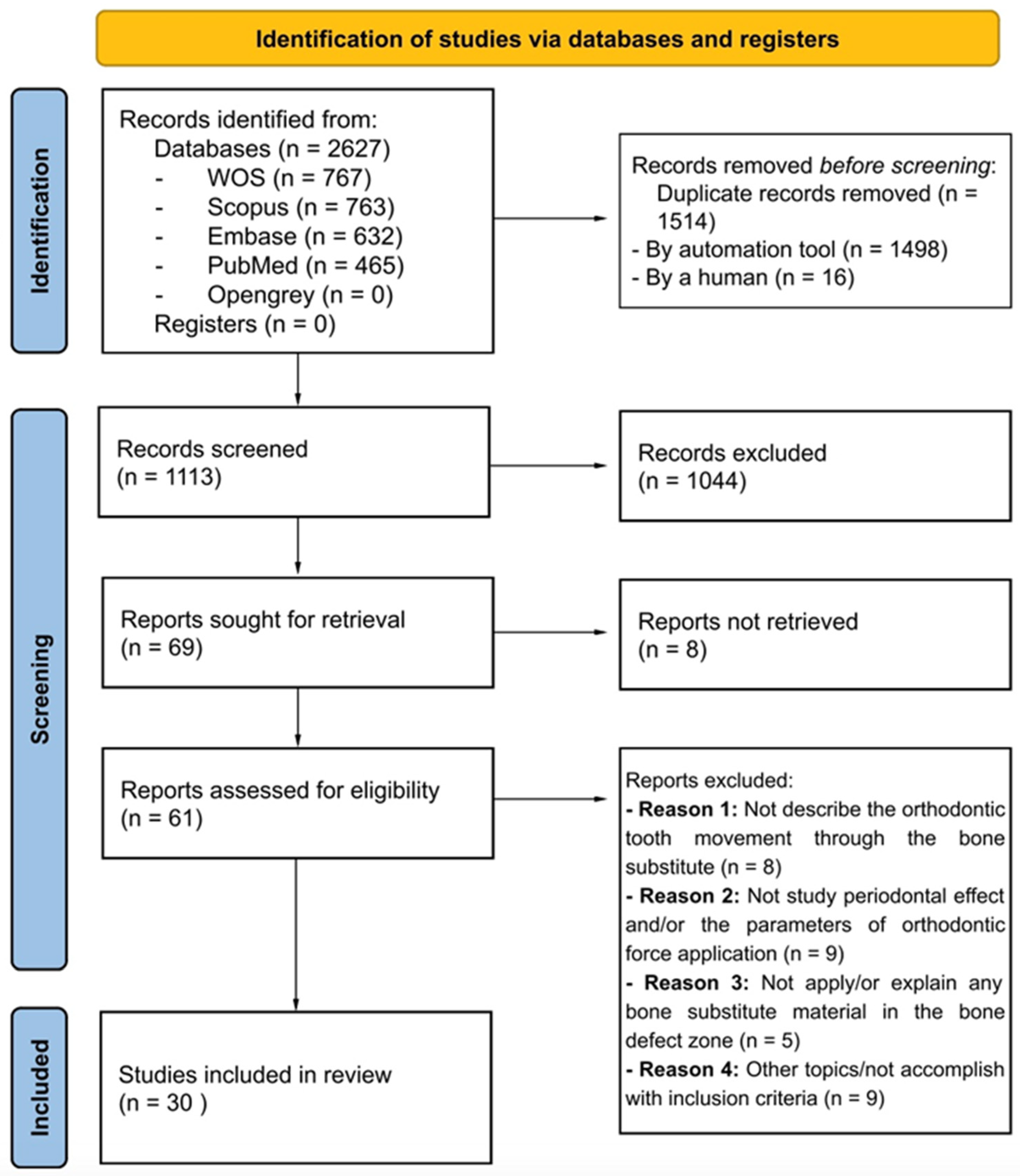
3.1. Literature Search and Screening Process
3.2. Description of the Included Studies
3.2.1. Study Design
3.2.2. Sample Characteristics
3.2.3. Type of Bone Defects
3.2.4. Type of Graft Materials
3.2.5. Parameters of Orthodontic Tooth Movements
- Localization of orthodontic movement
- Timing of orthodontic force application
- Direction of orthodontic force
- Magnitude of orthodontic force
- Mode of orthodontic force application
- Total duration of orthodontic tooth movement
3.2.6. Biological Repercussions on the Periodontium Complex and Methods of Analysis
- Bone response
- Clinical attachment level (CAL)
- Probing pocket depth (PPD)
- Root integrity/resorption
- Methods of analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current Trends and Future Perspectives of Bone Substitute Materials—From Space Holders to Innovative Biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Zhang, H.; Xu, W.; Zhang, C.; Yang, Y.; Zheng, X.; Xu, J. Bone Graft Materials for Alveolar Bone Defects in Orthodontic Tooth Movement. Tissue Eng. Part B Rev. 2022, 28, 35–51. [Google Scholar] [CrossRef]
- Sendyk, M.; Linhares, D.S.; Pannuti, C.M.; de Paiva, J.B.; Neto, J.R. Effect of Orthodontic Treatment on Alveolar Bone Thickness in Adults: A Systematic Review. Dent. Press J. Orthod. 2019, 24, 34–45. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.X.; Nie, J.; Mi, C.B.; Li, Y.M. Interactions between Orthodontic Treatment and Gingival Tissue. Chin. J. Dent. Res. 2023, 26, 11–18. [Google Scholar] [CrossRef]
- Yassir, Y.A.; McIntyre, G.T.; Bearn, D.R. Orthodontic Treatment and Root Resorption: An Overview of Systematic Reviews. Eur. J. Orthod. 2021, 43, 442–456. [Google Scholar] [CrossRef]
- Klein, Y.; Kunthawong, N.; Fleissig, O.; Casap, N.; Polak, D.; Chaushu, S. The Impact of Alloplast and Allograft on Bone Homeostasis: Orthodontic Tooth Movement into Regenerated Bone. J. Periodontol. 2020, 91, 1067–1075. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Kyomen, S.; Tanne, K. Biologic Responses of Autogenous Bone and Beta-Tricalcium Phosphate Ceramics Transplanted into Bone Defects to Orthodontic Forces. Cleft Palate-Craniofac. J. 1996, 33, 277–283. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Kniha, K.; Magnuska, Z.; Chhatwani, S.; Hermanns-Sachweh, B.; Gremse, F.; Hölzle, F.; Danesh, G.; Modabber, A. Development of Root Resorption during Orthodontic Tooth Movement after Cleft Repair Using Different Grafting Materials in Rats. Clin. Oral Investig. 2022, 26, 5809–5821. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, T.; Wu, G.; Li, W.; Feng, X.; Pathak, J.L.; Shi, J. BMP2-Functionalized Biomimetic Calcium Phosphate Graft Promotes Alveolar Defect Healing during Orthodontic Tooth Movement in Beagle Dogs. Front. Bioeng. Biotechnol. 2020, 8, 517. [Google Scholar] [CrossRef]
- Li, Y.-H.; Zhang, F.-F.; Bao, S.-J.; Wei, B.; Gong, Y. Study on Periodontal Responses on the Compression Side during Early Tooth Movement into Alveolar Defect Regenerated by a Tissue Engineering Bone. Shanghai Kou Qiang Yi Xue Shanghai J. Stomatol. 2018, 27, 461–466. [Google Scholar]
- Ma, Z.; Wang, Z.; Zheng, J.; Chen, X.; Xu, W.; Zou, D.; Zhang, S.; Yang, C. Timing of Force Application on Buccal Tooth Movement into Bone-Grafted Alveolar Defects: A Pilot Study in Dogs. Am. J. Orthod. Dentofac. Orthop. 2021, 159, e123–e134. [Google Scholar] [CrossRef]
- Machibya, F.M.; Zhuang, Y.; Guo, W.; You, D.; Lin, S.; Wu, D.; Chen, J. Effects of Bone Regeneration Materials and Tooth Movement Timing on Canine Experimental Orthodontic Treatment. Angle Orthod. 2018, 88, 171–178. [Google Scholar] [CrossRef]
- Mao, L.-X.; Shen, G.-F.; Fang, B.; Xia, Y.-H.; Ma, X.-H.; Wang, B. Bone Grafting, Corticotomy, and Orthodontics: Treatment of Cleft Alveolus in a Chinese Cohort. Cleft Palate-Craniofac. J. 2013, 50, 662–670. [Google Scholar] [CrossRef]
- Ru, N.; Liu, S.S.-Y.; Bai, Y.; Li, S.; Liu, Y.; Zhou, G. Microarchitecture and Biomechanical Evaluation of BoneCeramic Grafted Alveolar Defects during Tooth Movement in Rat. Cleft Palate-Craniofac. J. Off. Publ. Am. Cleft Palate-Craniofac. Assoc. 2018, 55, 798–806. [Google Scholar] [CrossRef]
- Ru, N.; Liu, S.S.-Y.; Bai, Y.; Li, S.; Liu, Y.; Wei, X. BoneCeramic Graft Regenerates Alveolar Defects but Slows Orthodontic Tooth Movement with Less Root Resorption. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 523–532. [Google Scholar] [CrossRef]
- Ru, N.; Liu, S.S.-Y.; Bai, Y.; Li, S.; Liu, Y.; Zhou, G. In Vivo Micro–Computed Tomography Evaluation of BoneCeramic Grafted Alveolar Defects during Orthodontic Tooth Movement. Angle Orthod. 2016. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Li, R.; Chen, Z.; Huang, Y.; Chen, Z. Biological Effects of Orthodontic Tooth Movement into the Grafted Alveolar Cleft. J. Oral Maxillofac. Surg. 2018, 76, 605–615. [Google Scholar] [CrossRef]
- Wang, L.; Hou, H.; Yu, S.; Guan, A.; Liao, Y. The Histological Study of Orthodontic Force on the Periodontal Tissues Regenerated by Nano Bioceramics in Beagle Dogs. In Proceedings of the 2nd International Conference on Biomedical and Biological Engineering 2017 (BBE 2017), Guilin, China, 26–28 May 2017; Atlantis Press: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Zhang, D.; Chu, F.; Yang, Y.; Xia, L.; Zeng, D.; Uludağ, H.; Zhang, X.; Qian, Y.; Jiang, X. Orthodontic Tooth Movement in Alveolar Cleft Repaired with a Tissue Engineering Bone: An Experimental Study in Dogs. Tissue Eng. Part A 2011, 17, 1313–1325. [Google Scholar] [CrossRef]
- Zhang, F.-F.; Bao, S.-J.; Ye, S.-J.; Wei, B.; Gong, Y. Study of the Timing of Tooth Movement after Repair of Alveolar Bone Defects by Rabbit BMSCs Combined with Beta-TCP. Shanghai Kou Qiang Yi Xue Shanghai J. Stomatol. 2019, 28, 231–236. [Google Scholar]
- Zhou, J.; Shu, R.; Gong, Y.; Xie, Y. Therapeutic Effect of Orthodontic Intrusion Combined with Periodontal Regenerative Surgery in the Treatment of Pathologic Migration of Upper Incisors. J. Shanghai Jiaotong Univ. (Med. Sci.) 2018, 38, 536–540. [Google Scholar]
- Kawamoto, T.; Motohashi, N.; Kitamura, A.; Baba, Y.; Suzuki, S.; Kuroda, T. Experimental Tooth Movement into Bone Induced by Recombinant Human Bone Morphogenetic Protein-2. Cleft Palate-Craniofac. J. 2003, 40, 538–543. [Google Scholar] [CrossRef]
- Kawamoto, T.; Motohashi, N.; Kitamura, A.; Baba, Y.; Takahashi, K.; Suzuki, S.; Kuroda, T. A Histological Study on Experimental Tooth Movement into Bone Induced by Recombinant Human Bone Morphogenetic Protein-2 in Beagle Dogs. Cleft Palate-Craniofac. J. 2002, 39, 439–448. [Google Scholar] [CrossRef]
- Tanimoto, K.; Sumi, K.; Yoshioka, M.; Oki, N.; Tanne, Y.; Awada, T.; Kato, Y.; Sugiyama, M.; Tanne, K. Experimental Tooth Movement into New Bone Area Regenerated by Use of Bone Marrow–Derived Mesenchymal Stem Cells. Cleft Palate-Craniofac. J. 2015, 52, 386–394. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Kniha, K.; Magnuska, Z.; Hermanns-Sachweh, B.; Gremse, F.; Hölzle, F.; Danesh, G.; Modabber, A. Evaluation of Different Grafting Materials for Alveolar Cleft Repair in the Context of Orthodontic Tooth Movement in Rats. Sci. Rep. 2021, 11, 13586. [Google Scholar] [CrossRef]
- Reichert, C.; Wenghöfer, M.; Götz, W.; Jäger, A. Pilot Study on Orthodontic Space Closure after Guided Bone Regeneration. J. Orofac. Orthop. Fortschritte Kieferorthopädie 2011, 72, 45–50. [Google Scholar] [CrossRef]
- Klein, Y.; Fleissig, O.; Stabholz, A.; Chaushu, S.; Polak, D. Bone Regeneration with Bovine Bone Impairs Orthodontic Tooth Movement despite Proper Osseous Wound Healing in a Novel Mouse Model. J. Periodontol. 2019, 90, 189–199. [Google Scholar] [CrossRef]
- Ahn, H.-W.; Ohe, J.-Y.; Lee, S.-H.; Park, Y.-G.; Kim, S.-J. Timing of Force Application Affects the Rate of Tooth Movement into Surgical Alveolar Defects with Grafts in Beagles. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 486–495. [Google Scholar] [CrossRef]
- Lee, K.-B.; Lee, D.-Y.; Ahn, H.-W.; Kim, S.-H.; Kim, E.-C.; Roitman, I. Tooth Movement out of the Bony Wall Using Augmented Corticotomy with Nonautogenous Graft Materials for Bone Regeneration. BioMed Res. Int. 2014, 2014, 347508. [Google Scholar] [CrossRef]
- Cardaropoli, D.; Re, S.; Manuzzi, W.; Gaveglio, L.; Cardaropoli, G. Bio-Oss Collagen and Orthodontic Movement for the Treatment of Infrabony Defects in the Esthetic Zone. Int. J. Periodont. Restor. Dent. 2006, 26, 553–559. [Google Scholar]
- Oltramari, P.V.P.; Navarro, R.d.L.; Henriques, J.F.C.; Taga, R.; Cestari, T.M.; Ceolin, D.S.; Janson, G.; Granjeiro, J.M. Orthodontic Movement in Bone Defects Filled with Xenogenic Graft: An Experimental Study in Minipigs. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 302.e10–302.e17. [Google Scholar] [CrossRef]
- Araujo, M.G.; Carmagnola, D.; Berglundh, T.; Thilander, B.; Lindhe, J. Orthodontic Movement in Bone Defects Augmented with Bio-OssR®: An Experimental Study in Dogs. J. Clin. Periodontol. 2001, 28, 73–80. [Google Scholar] [CrossRef]
- Yılmaza, S.; Kılıçb, A.R.; Kelesc, A.; Efeoğlud, E. Reconstruction of an Alveolar Cleft for Orthodontic Tooth Movement. Am. J. Orthod. Dentofac. Orthop. 2000, 117, 156–163. [Google Scholar] [CrossRef]
- Attia, M.S.; Shoreibah, E.A.; Ibrahim, S.A.; Nassar, H.A. Regenerative Therapy of Osseous Defects Combined with Orthodontic Tooth Movement. J. Int. Acad. Periodontol. 2012, 14, 17–25. [Google Scholar]
- Fung, K.; Chandhoke, T.K.; Uribe, F.; Schincaglia, G.P. Periodontal Regeneration and Orthodontic Intrusion of a Pathologically Migrated Central Incisor Adjacent to an Infrabony Defect. J. Clin. Orthod. 2012, 46, 417–423. [Google Scholar]
- Giannoudis, P.V.; Jones, E.; Einhorn, T.A. Fracture Healing and Bone Repair. Injury 2011, 42, 549–550. [Google Scholar] [CrossRef]
- Perry, C.R. Bone Repair Techniques, Bone Graft, and Bone Graft Substitutes. Clin. Orthop. Relat. Res. 1999, 360, 71–86. [Google Scholar] [CrossRef]
- Van der Stok, J.; Van Lieshout, E.M.M.; El-Massoudi, Y.; Van Kralingen, G.H.; Patka, P. Bone Substitutes in the Netherlands—A Systematic Literature Review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [CrossRef]
- Muschler, G.F.; Raut, V.P.; Patterson, T.E.; Wenke, J.C.; Hollinger, J.O. The Design and Use of Animal Models for Translational Research in Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part B Rev. 2010, 16, 123–145. [Google Scholar] [CrossRef]
- Hara, Y.; Murakami, T.; Kajiyama, K.; Maeda, K.; Akamine, A.; Nagamine, N.; Miyatake, S.; Abe, T.; Azemoto, Y.; Aono, M. Application of calcium phosphate ceramics to periodontal therapy. 8. Effects of orthodontic force on repaired bone with hydroxyapatite. Nihon Shishubyo Gakkai Kaishi 1989, 31, 224–234. [Google Scholar] [CrossRef][Green Version]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Laurencin, C.; Khan, Y.; El-Amin, S.F. Bone Graft Substitutes. Expert Rev. Med. Devices 2006, 3, 49–57. [Google Scholar] [CrossRef]
- Szabó, G.; Huys, L.; Coulthard, P.; Maiorana, C.; Garagiola, U.; Barabás, J.; Németh, Z.; Hrabák, K.; Suba, Z. A Prospective Multicenter Randomized Clinical Trial of Autogenous Bone versus Beta-Tricalcium Phosphate Graft Alone for Bilateral Sinus Elevation: Histologic and Histomorphometric Evaluation. Int. J. Oral Maxillofac. Implant. 2005, 20, 371–381. [Google Scholar]
- Hsu, Y.-T.; Wang, H.-L. How to Select Replacement Grafts for Various Periodontal and Implant Indications. Clin. Adv. Periodont. 2013, 3, 167–179. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A Comprehensive Review of Biodegradable Synthetic Polymer-Ceramic Composites and Their Manufacture for Biomedical Applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef]
- Cope, J.B.; Samchukov, M.L. Regenerate Bone Formation and Remodeling during Mandibular Osteodistraction. Angle Orthod. 2000, 70, 99–111. [Google Scholar]
- Uckan, S.; Guler, N.; Arman, A.; Mutlu, N. Mandibular Midline Distraction Using a Simple Device. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, D.A.; Wolford, L.M. Long-Term Evaluation of the Use of Coralline Hydroxyapatite in Orthognathic Surgery. J. Oral Maxillofac. Surg. 1998, 56, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Maltha, J.C.; Kuijpers-Jagtman, A.M. Optimum Force Magnitude for Orthodontic Tooth Movement: A Systematic Literature Review. Angle Orthod. 2003, 73, 86–92. [Google Scholar]
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and Biological Responses of Periodontium in Orthodontic Tooth Movement: Up-Date in a New Decade. Int. J. Oral Sci. 2021, 13, 20. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone Graft Materials. An Overview of the Basic Science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Rokn, A.R.; Khodadoostan, M.A.; Ghahroudi, A.A.R.R.; Motahhary, P.; Fard, M.J.K.; Bruyn, H.D.; Afzalifar, R.; Soolar, E.; Soolari, A. Bone Formation with Two Types of Grafting Materials: A Histologic and Histomorphometric Study. Open Dent. J. 2011, 5, 96–104. [Google Scholar] [CrossRef]
- Artzi, Z.; Tal, H.; Dayan, D. Porous Bovine Bone Mineral in Healing of Human Extraction Sockets. Part 1: Histomorphometric Evaluations at 9 Months. J. Periodontol. 2000, 71, 1015–1023. [Google Scholar] [CrossRef]
- Cate, R.T. Oral Histology: Development, Structure and Function, 5th ed.; Mosby: Maryland Heights, MO, USA, 1998. [Google Scholar]
- Liu, T.; Zheng, Y.; Wu, G.; Wismeijer, D.; Pathak, J.L.; Liu, Y. BMP2-Coprecipitated Calcium Phosphate Granules Enhance Osteoinductivity of Deproteinized Bovine Bone, and Bone Formation during Critical-Sized Bone Defect Healing. Sci. Rep. 2017, 7, 41800. [Google Scholar] [CrossRef]
- Kulak, C.A.; Dempster, D.W. Bone histomorphometry: A concise review for endocrinologists and clinicians. Arq. Bras. Endocrinol. Metabol. 2010, 54, 87–98. [Google Scholar] [CrossRef]
- Rentsch, C.; Schneiders, W.; Manthey, S.; Rentsch, B.; Rammelt, S. Comprehensive histological evaluation of bone implants. Biomatter 2014, 4, e27993. [Google Scholar] [CrossRef]
- Vandeweghe, S.; Coelho, P.G.; Vanhove, C.; Wennerberg, A.; Jimbo, R. Utilizing micro-computed tomography to evaluate bone structure surrounding dental implants: A comparison with histomorphometry. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1259–1266. [Google Scholar] [CrossRef]
- Shanbhag, S.; Suliman, S.; Pandis, N.; Stavropoulos, A.; Sanz, M.; Mustafa, K. Cell therapy for orofacial bone regeneration: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 162–182. [Google Scholar] [CrossRef]
- Ren, Y.; Vissink, A. Cytokines in crevicular fluid and orthodontic tooth movement. Eur. J. Oral Sci. 2008, 116, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.P.; Kao, D.W.; Kim, D.M.; Uzel, N.G. Anatomy of the Periodontium. In Carranza’s Clinical Periodontology; Elsevier: St. Louis, MO, USA, 2015. [Google Scholar]
| Database | Search Strategy |
|---|---|
| Web of Science | (TS=((“orthodontic movement” OR “tooth movement” OR “orthodontic treatment”))) AND TS=((“bone defect*” OR “alveolar defect*” OR “osseus defect*” OR “bone graft” OR “bone regeneration” OR “alveolar cleft”)) |
| Scopus | (TITLE-ABS-KEY ((“orthodontic movement” OR “tooth movement” OR “orthodontic treatment”)) AND TITLE-ABS-KEY ((“bone defect*” OR “alveolar defect*” OR “osseus defect*” OR “bone graft” OR “bone regeneration” OR “alveolar cleft”))) |
| Embase | (“orthodontic movement” OR “tooth movement” OR “orthodontic treatment”) AND (“bone defect*” OR “alveolar defect*” OR “osseus defect*” OR “bone graft” OR “bone regeneration” OR “alveolar cleft”) |
| PubMed | (“orthodontic movement”[All Fields] OR “tooth movement”[All Fields] OR “orthodontic treatment”[All Fields]) AND (“bone defect*”[All Fields] OR “alveolar defect*”[All Fields] OR “osseus defect*”[All Fields] OR “bone graft”[All Fields] OR “bone regeneration”[All Fields] OR “alveolar cleft”[All Fields]) |
| Opengrey | (orthodontic movement OR tooth movement OR orthodontic treatment) AND (bone defect OR alveolar defect OR osseus defect OR bone graft OR bone regeneration OR alveolar cleft) |
| Authors/Year | Study Design | Species Age Gender | Type of Defect | Size Localization | Regeneration Materials |
|---|---|---|---|---|---|
| Ahn HW et al. (2014) [30] | Experimental | Dog 18 to 24 m 12 M | Extraction socket |
| DBB (Bio-Oss) and DBM (OrthoBlast II) |
| Araújo M et al. (2001) [34] | Experimental | Dog 1 y 5 N/R | Extraction socket |
| DBB (BioOssA) |
| Attia MS et al. (2012) [36] | Experimental | Human 25 to 48 y 10 F, 5 M | Infrabony defects |
| BG (Bio-Glass) |
| Cardaropoli D et al. (2006) [32] | Case report | Human N/R 3 M | Infrabony defects |
| DBB (Bio-Oss) |
| Fung K et al. (2012) [37] | Case report | Human 68 y 1 F | Infrabony defects |
| EMD (Emdogain) and BCP |
| Hossain M et al. (1996) [9] | Experimental | Dog 1 y 9 N/R | Extraction socket |
| AB and β-TCP |
| Jiang S et al. (2020) [11] | Experimental | Dog 1 y 9 M | Extraction socket |
| BioCaP and DBB |
| Kawamoto T et al. (2003) [24] | Experimental | Dog 1.6 to 2.6 y 8 F | Extraction socket |
| rhBMP-2 with PGS |
| Kawamoto T et al. (2002) [25] | Experimental | Dog 1 y 5 m to 2 y 3 m 8 F | Extraction socket |
| rhBMP-2with PGS |
| Klein Y et al. (2019) [29] | Experimental | Mouse 6/7 w 44 M | Extraction socket |
| BB |
| Klein Y et al. (2020) [7] | Experimental | Mouse 6/7 w 54 M | Extraction socket |
| AG and β-TCP |
| Lee KB et al. (2014) [31] | Experimental | Dog 1/2 y 6 M | Periodontal defects |
| DBB, (Bio-Oss), IB, SB, BCP |
| Li YH et al. (2018) [12] | Experimental | Rabbit 5 to 6 m 30 N/R | Extraction socket |
| BMSCs and β-TCP |
| Ma Z et al. (2021) [13] | Experimental | Dog 1.5 y 6 M | Dehiscencetype defects |
| DBB (Bio-Oss) |
| Machibya FM et al. (2018) [14] | Experimental | Dog 18 m 6 M | Extraction socket |
| DBB (Bio-Oss) and β-TCP |
| Mao L et al. (2013) [15] | Observational | Human 18.3 ± 4.2 y 30 N/R | Unilateral cleft lip and palate |
| AB |
| Moehlhenrich SC et al. (2021) [27] | Experimental | Rat 8 w 21 M | Alveolar cleft |
| AB, XHB, β-TCP, and HA |
| Moehlhenrich SC et al. (2022) [10] | Experimental | Rat 8 w 21 M | Alveolar cleft |
| AB, XHB, β-TCP, and HA |
| Oltramari PVP et al. (2007) [33] | Experimental | Minipig 12 m 6 M | Extraction socket |
| DBB, BMP, and HA |
| Reichert C et al. (2011) [28] | Case report | Human 11.6 y, 13.10 y, 11.2 y 1 F, 2 M | Extraction socket |
| NanoBone |
| Ru N et al. (August 2016) [18] | Experimental | Rat 5 w 60 M | Extraction socket |
| BCP (bone ceramic), DBB(BioOss) |
| Ru N et al. (April 2016) [17] | Experimental | Rat 5 w 60 M | Extraction socket |
| BCP (bone ceramic); DBB(BioOss) |
| Ru N et al. (2018) [16] | Experimental | Rat 5 w 60 M | Extraction socket |
| BCP (bone ceramic); DBB(BioOss) |
| Sun J et al. (2018) [19] | Experimental | Rat 8 w 39 M | Alveolar cleft |
| AB |
| Tanimoto K et al. (2015) [26] | Experimental | Dog 3 m 3 F | Alveolar cleft |
| BMSCs and HA |
| Wang L Lei et al. (2017) [20] | Experimental | Dog 1.5 y 2 M | Alveolar bone defect |
| NBCP |
| Yilmaz S et al. (2000) [35] | Case report | Human 16 y 1 M | Unilateral cleft and palate |
| DFDBA and BG |
| Zhang D et al. (2011) [21] | Experimental | Dog 24 w 7 M | Alveolar cleft |
| BMSCs and β-TCP, β-TCP, AB |
| Zhang FF et al. (2019) [22] | Experimental | Rabbit 20 to 24 y 40 N/R | Extraction socket |
| BMSCs and β-TCP |
| Zhou J et al. (2018) [23] | Case report | Human 38.4 y 7 F, 2 M | Vertical bone defect |
| DBB (Bio-Oss) |
| Dog | Human | Rat | Mouse | Rabbit | Minipig | |
|---|---|---|---|---|---|---|
| Number of studies | 12 | 7 | 6 | 2 | 2 | 1 |
| Sample (n) | 84 | 62 | 261 | 98 | 70 | 6 |
| Male | 51 | 13 | 261 | 98 | 0 | 6 |
| Female | 15 | 19 | 0 | 0 | 0 | 0 |
| N/R | 18 | 30 | 0 | 0 | 70 | 0 |
| Age | 3–27 months | 11.2–68 years | 5–12 weeks | 6–7 weeks | 20–24 weeks | 12 months |
| Authors/Year | Localization of OTM | Time after Surgery/Treatment | Characteristics of the Force | Total Duration of OTM | Amount of OTM |
|---|---|---|---|---|---|
| Ahn HW et al. (2014) [30] | Between Mx-C and Mx-SPM |
|
|
| 1.75 to 3.44 mm |
| Araújo M et al. (2001) [34] | Between Mb TPM and Mb FM |
|
|
| 3.85 ± 57 mm |
| Attia MS et al. (2012) [36] | N/R |
|
|
| N/R |
| Cardaropoli D et al. (2006) [32] | Mx CI |
|
|
| N/R |
| Fung K et al. (2012) [37] | Between Mx CI |
|
|
| 1 mm |
| Hossain M et al. (1996) [9] | Between Mx C and CI |
|
|
| N/R |
| Jiang S et al. (2020) [11] | Between Mx SPM and C |
|
|
| DBB group: 3.59 ± 1.25 BioCap group: 2.90 ± 0.84 |
| Kawamoto T et al. (2003) [24] | Between Max SPM and C |
|
|
| 2 mm |
| Kawamoto T et al. (2002) [25] | Max 2PM |
|
|
| 2 mm |
| Klein Y et al. (2019) [29] | Between Mx SM and I |
|
|
| 550.36 μm ± 101.52 |
| Klein Y et al. (2020) [7] | Between Mx SM and I |
|
|
| β-TCP group: 707.3 ± 30.6 μm AG group 648.3 ± 31.6 μm |
| Lee KB et al. (2014) [31] | Between Mx SPM and TPM and Mb SPM and TPM |
|
|
| DBBM group: 20.81 ± 8.07° IB group: 16.08 ± 4.14° SB group: 27.26 ± 7.27° |
| Li YH et al. (2018) [12] | Between Mx I and SM |
|
|
| BMSCs + β-TCP group: 3.17 ± 0.26 β-TCP group: 2.79 ± 0.12 |
| Ma Z et al. (2021) [13] | Between Mx C and FPM |
|
|
| Expansion and buccal tipping: Immediately force application group: 2.42 mm and 9.03 ± 1.02 4 w after surgery force application group: 1.25 mm and 5.32 ± 2.19 8 w after surgery force applicationgroup: 1.62 mm and 3.24 ± 1.27 |
| Machibya FM et al. (2018) [14] | Between Mx and MbC and SPM |
|
|
| Bio-Oss group 4.22 mm β-TCP group: 4.76 mm |
| Mao L et al. (2013) [15] | Between Mc C and CI |
|
|
| N/R |
| Moehlhenrich SC et al. (2021) [27] | Between Mx FM and I |
|
|
| N/R |
| Moehlhenrich SC et al. (2022) [10] | Between Mx FM and I |
|
|
| SB group: 0.82 ± 0.72 mm XHB group: 0.78 ± 0.69 mm AB group: 0.67 ± 0.27 mm |
| Oltramari PVP et al. (2007) [33] | Between Mx and Mb FM and DTM |
|
|
| 4 mm |
| Reichert C et al. (2011) [28] | Between Mx SPM, between Mx FPM, between Mb FPM |
|
|
| N/R |
| Ru N et al. (August 2016) [18] | Between Mx SM and I |
|
|
| BCP with a lower amount of OTM than DBB |
| Ru N et al. (April 2016) [17] | Between Mx SM and I |
|
|
| BCP with a lower amount of OTM than DBB |
| Ru N et al. (2018) [16] | Between Mx SM and I |
|
|
| BCP with a lower amount of OTM than DBB |
| Sun J et al. (2018) [19] | Between Mx SM and I |
|
|
| N/R |
| Tanimoto K et al. (2015) [26] | Between Mx SI and C |
|
|
| 6 mm |
| Wang L Lei et al. (2017) [20] | Between Mx and Mb TI and C |
|
|
| N/R |
| Yilmaz S et al. (2000) [35] | Between Mx LI and C |
|
|
| N/R |
| Zhang D et al. (2011) [21] | Between Mx LI and C |
|
|
| bMSCs/β-TCP group: 5.345 ± 0.936 mm β-TCP group: 6.986 ± 1.412 mm AB group: 4.665 ± 0.483 mm |
| Zhang FF et al. (2019) [22] | Between Mb SM and FPM |
|
| 4 w |
|
| Zhou J et al. (2018) [23] | Mx I |
|
| 11.3 m | N/R |
| Authors/Year | Bone Formation/Resorption | Clinical Attachment Level | Roots Integrity/Resorption | Probing Pocket Depth | Methods of Analysis |
|---|---|---|---|---|---|
| Ahn HW et al. (2014) [30] | Increased BF | N/R | N/R | N/R |
|
| Araújo M et al. (2001) [34] | Increased BF | N/R | Minor RR | N/R |
|
| Attia MS et al. (2012) [36] | Increased BF | Increased clinical attachment | N/R | N/R |
|
| Cardaropoli D et al. (2006) [32] | Increased BF | Increased CAL | N/R | Decreased PPD |
|
| Fung K et al. (2012) [37] | Increased BF | Increased CAL | No RR | Decreased PPD |
|
| Hossain M et al. (1996) [9] | Increased BF | Increased attachments of the PDL fibers | Minor RR | N/R |
|
| Jiang S et al. (2020) [11] | Increased BF | N/R | Increased RR in BioCap | Increased PPD |
|
| Kawamoto T et al. (2003) [24] | Increased BF | N/R | Partial cementum resorption | N/R |
|
| Kawamoto T et al. (2002) [25] | Increased BF | N/R | Negligible cementum resorption | N/R |
|
| Klein Y et al. (2019) [29] | Increased BF | N/R | N/R | N/R |
|
| Klein Y et al. (2020) [7] | Increased BF | N/R | N/R | N/R |
|
| Lee KB et al. (2014) [31] | Increased BF | N/R | Partial cementum resorption | Increased PD |
|
| Li YH et al. (2018) [12] | Increased BF (better in BMSCs +β-TCP than β-TCP) | N/R | No RR | N/R |
|
| Ma Z et al. (2021) [13] | Increased BF | N/R | N/R | N/R |
|
| Machibya FM et al. (2018) [14] | Increased BF | N/R | N/R | N/R |
|
| Mao L et al. (2013) [15] | Decreased BF in less than 25% of the sample | N/R | Slight RR | N/R |
|
| Moehlhenrich SC et al. (2021) [27] | Increased BF (highest in the XHB group and lowest in the SB group) | N/R | N/R | N/R |
|
| Moehlhenrich SC et al. (2022) [10] | N/R | N/R | RR in all groups | N/R |
|
| Oltramari PVP et al. (2007) [33] | BR and BF were balanced | N/R | Slight RR | N/R |
|
| Reichert C et al. (2011) [28] | N/R | N/R | No RR | N/R |
|
| Ru N et al. (August 2016) [18] | BCP with more BF than DBB | N/R | N/R | N/R |
|
| Ru N et al. (April 2016) [17] | BCP with more BF than DBB | N/R | BCP with less RR than DBB | N/R |
|
| Ru N et al. (2018) [16] | BCP with more BF than DBB | N/R | BCP with less RR than DBB | N/R |
|
| Sun J et al. (2018) [19] | Increased BF | N/R | N/R | N/R |
|
| Tanimoto K et al. (2015) [26] | Increased BF | N/R | No root resorption | N/R |
|
| Wang L Lei et al. (2017) [20] | No difference between newly formed periodontium and normal periodontal tissues | No difference between newly formed periodontium and normal periodontal tissues | No difference between newly formed periodontium and normal periodontal tissues | No difference between newly formed periodontium and normal periodontal tissues |
|
| Yilmaz S et al. (2000) [35] | Increased BF | No gums recessions | No RR | N/R |
|
| Zhang D et al. (2011) [21] | Increased BF (higher in BMSCs/β-TCP group than β-TCP group) | N/R | N/R | N/R |
|
| Zhang FF et al. (2019) [22] | N/R | N/R | N/R | N/R |
|
| Zhou J et al. (2018) [23] | Increased BF | Increased CAL | N/R | Decreased PPD |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdecchia, A.; Suárez-Fernández, C.; Miquel, A.; Bardini, G.; Spinas, E. Biological Effects of Orthodontic Tooth Movement on the Periodontium in Regenerated Bone Defects: A Scoping Review. Dent. J. 2024, 12, 50. https://doi.org/10.3390/dj12030050
Verdecchia A, Suárez-Fernández C, Miquel A, Bardini G, Spinas E. Biological Effects of Orthodontic Tooth Movement on the Periodontium in Regenerated Bone Defects: A Scoping Review. Dentistry Journal. 2024; 12(3):50. https://doi.org/10.3390/dj12030050
Chicago/Turabian StyleVerdecchia, Alessio, Carlota Suárez-Fernández, Andrea Miquel, Giulia Bardini, and Enrico Spinas. 2024. "Biological Effects of Orthodontic Tooth Movement on the Periodontium in Regenerated Bone Defects: A Scoping Review" Dentistry Journal 12, no. 3: 50. https://doi.org/10.3390/dj12030050
APA StyleVerdecchia, A., Suárez-Fernández, C., Miquel, A., Bardini, G., & Spinas, E. (2024). Biological Effects of Orthodontic Tooth Movement on the Periodontium in Regenerated Bone Defects: A Scoping Review. Dentistry Journal, 12(3), 50. https://doi.org/10.3390/dj12030050







