Novel Technique to Reconstruct Peri-Implant Keratinised Mucosa Width Using Xenogeneic Dermal Matrix. Clinical Case Series
Abstract
1. Introduction and Background
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
- Men and women older than 18 years of age;
- Good compliance to adhere to follow-up protocol and a willingness to commit to a long-term maintenance program after treatment;
- Good oral hygiene (full mouth plaque score, FMPS < 20%);
- PIKM-W either absent or less than 2 mm, measured with a UNC 15 periodontal probe;
- Planned implant placement in the area of keratinised soft-tissue augmentation.
- Contributing medical history in which any elective oral surgical intervention would be contraindicated;
- Smoking;
- Pregnant or lactating women;
- Presence of uncontrolled or untreated periodontal disease (full mouth bleeding score > 20%).
2.3. Presurgical Treatment
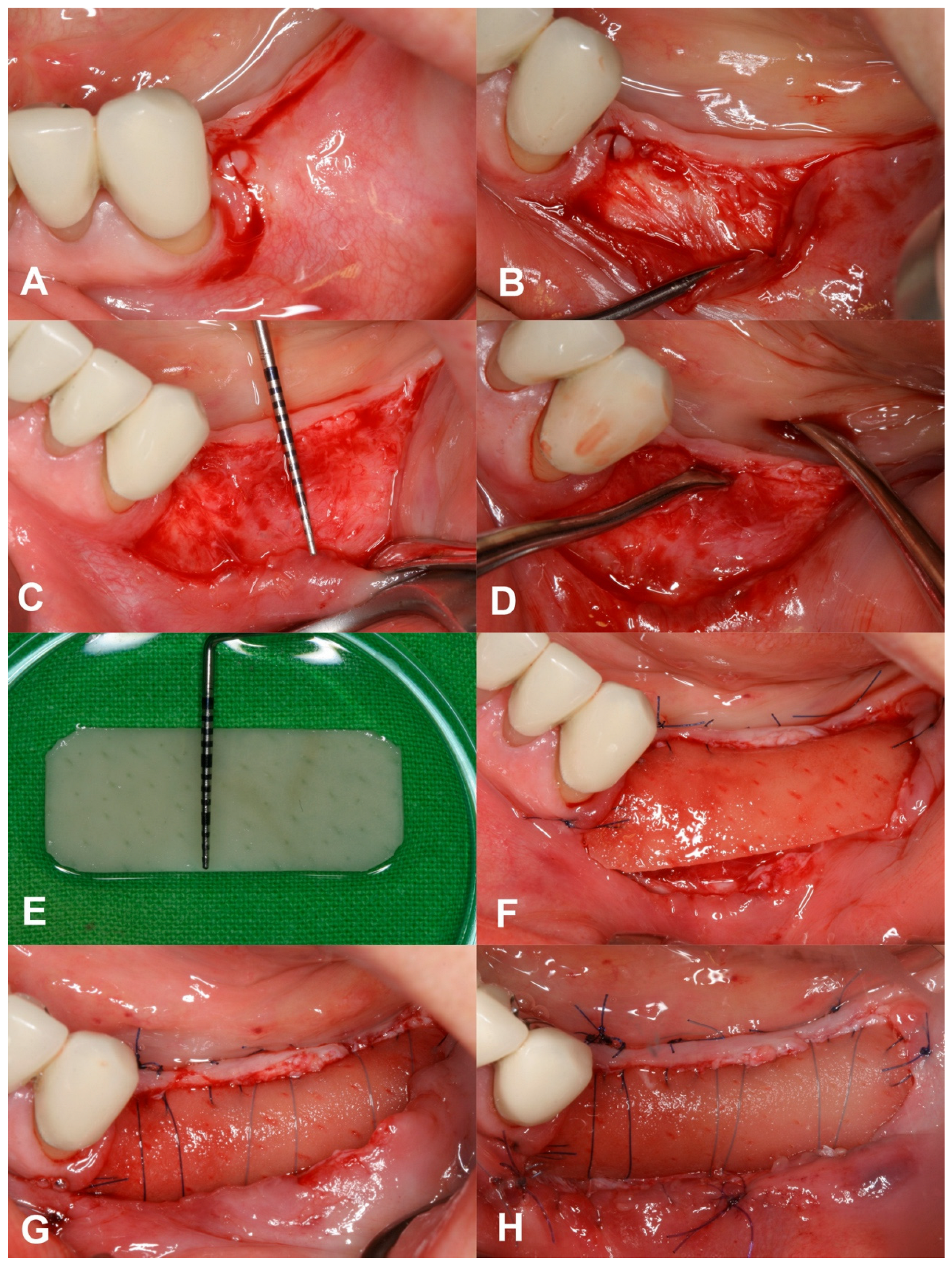
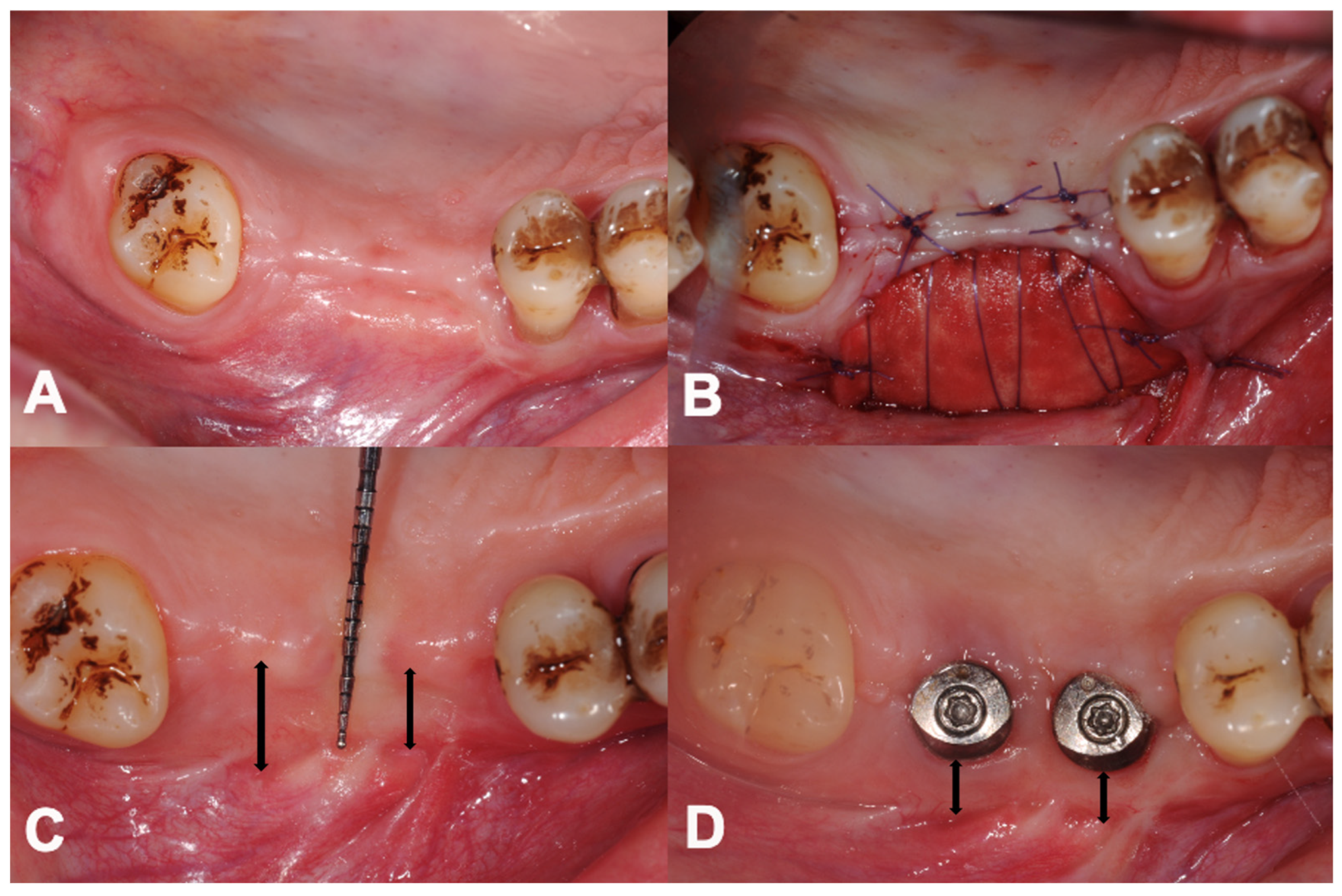
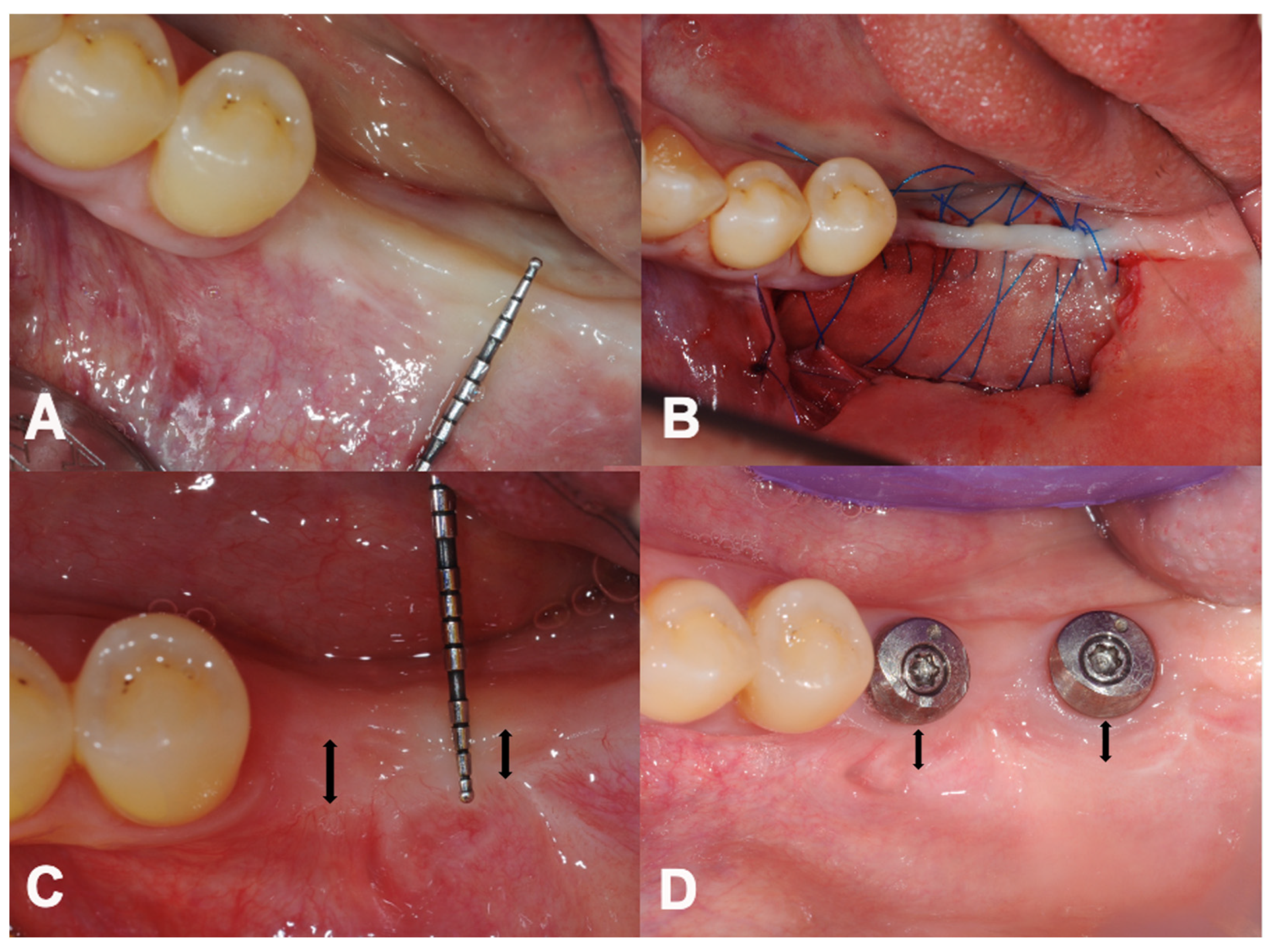
2.4. Surgical Treatment
2.5. Postsurgical Instructions and Infection Control
2.6. Outcome Variables
2.7. Statistical Analysis
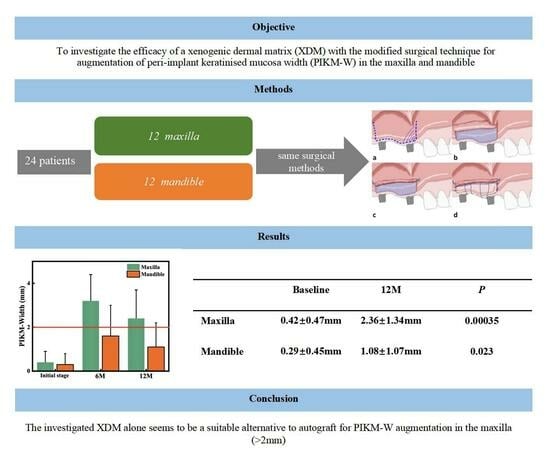
3. Results
3.1. Patient Demographics
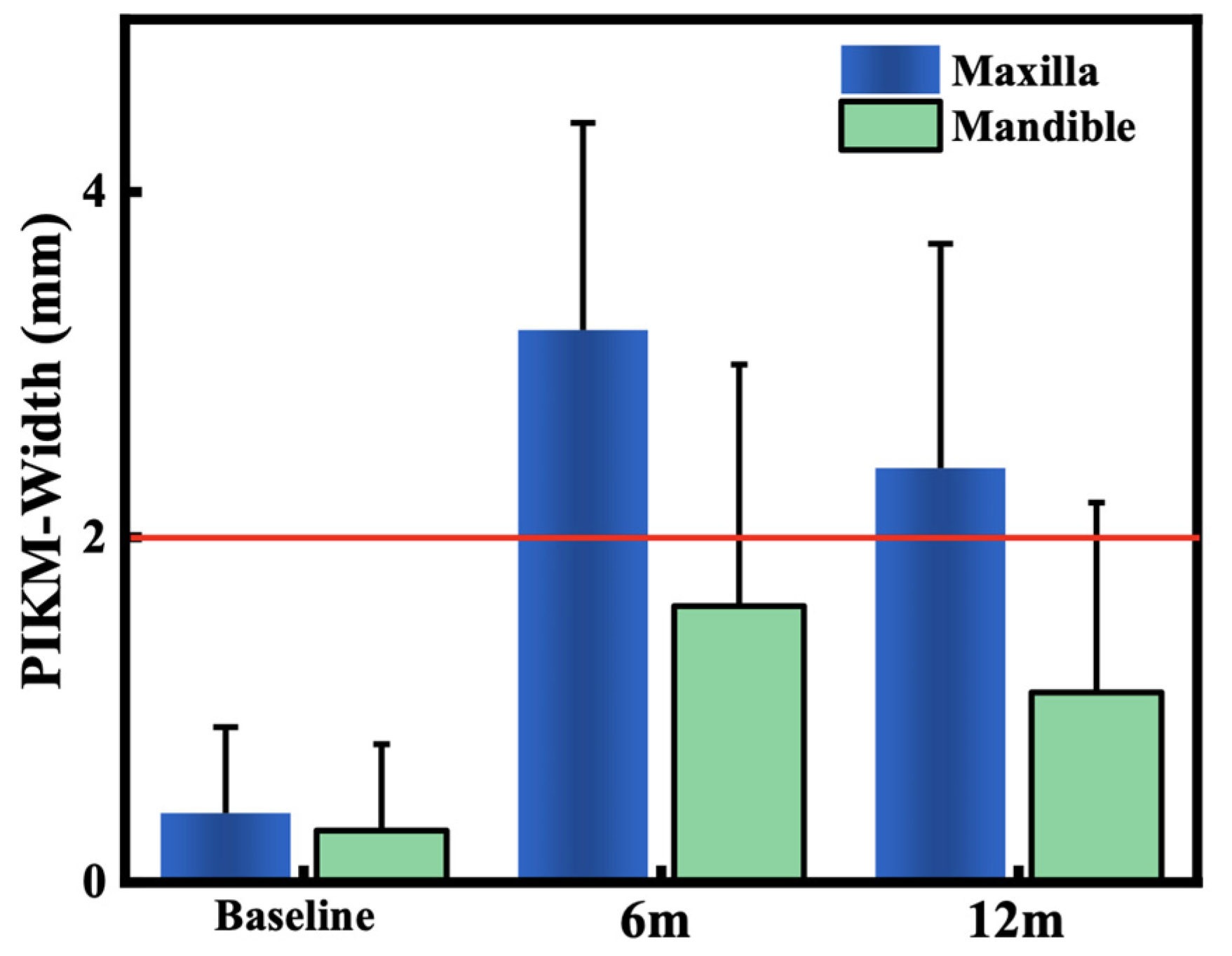
3.2. Primary Outcome: Changes in PIKM-W
3.3. Secondary Outcome: Dimension of Graft Remodelling
4. Discussion
5. Conclusions
- The investigated surgical technique using XDM was safe and significantly widened the PIKM in both jaws.
- The new PIKM formation surpassed the clinically desired 2 mm width in the maxilla. The mandibular sites presented with more variable success.
- The remodelling of the newly formed PIKM continued after 6 months.
- The investigated XDM alone could be considered as a suitable alternative to autograft to reconstruct an adequate amount of keratinised mucosa width in the maxilla.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of Soft Tissue Augmentation Procedures on Peri-Implant Health or Disease: A Systematic Review and Meta-Analysis. Clin. Oral Implants Res. 2018, 29 (Suppl. S15), 32–49. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A Systematic Review of the 5-Year Survival and Complication Rates of Implant-Supported Single Crowns. Clin. Oral Implants Res. 2008, 19, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-Implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V. An Insight into Peri-Implantitis: A Systematic Literature Review. Prim. Dent. J. 2013, 2, 69–73. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-Implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Almutairi, Z.; Amir-Rad, F.; Koleilat, M.; Tawse-Smith, A.; Ma, S.; Lin, L.; Alsabeeha, N.H.M. A Retrospective Analysis of Biological Complications of Dental Implants. Int. J. Dent. 2022, 2022, 1545748. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.F.; Tarnow, D. The Etiology of Hard- and Soft-Tissue Deficiencies at Dental Implants: A Narrative Review. J. Periodontol. 2018, 89 (Suppl. S1), S291–S303. [Google Scholar] [CrossRef]
- Wennström, J.L.; Bengazi, F.; Lekholm, U. The Influence of the Masticatory Mucosa on the Peri-Implant Soft Tissue Condition. Clin. Oral Implants Res. 1994, 5, 1–8. [Google Scholar] [CrossRef]
- Bouri, A.; Bissada, N.; Al-Zahrani, M.S.; Faddoul, F.; Nouneh, I. Width of Keratinized Gingiva and the Health Status of the Supporting Tissues around Dental Implants. Int. J. Oral Maxillofac. Implants 2008, 23, 323–326. [Google Scholar]
- Roccuzzo, M.; Grasso, G.; Dalmasso, P. Keratinized Mucosa around Implants in Partially Edentulous Posterior Mandible: 10-Year Results of a Prospective Comparative Study. Clin. Oral Implants Res. 2016, 27, 491–496. [Google Scholar] [CrossRef]
- Gharpure, A.S.; Latimer, J.M.; Aljofi, F.E.; Kahng, J.H.; Daubert, D.M. Role of Thin Gingival Phenotype and Inadequate Keratinized Mucosa Width (<2 mm) as Risk Indicators for Peri-Implantitis and Peri-Implant Mucositis. J. Periodontol. 2021, 92, 1687–1696. [Google Scholar] [PubMed]
- Kim, B.-S.; Kim, Y.-K.; Yun, P.-Y.; Yi, Y.-J.; Lee, H.-J.; Kim, S.-G.; Son, J.-S. Evaluation of Peri-Implant Tissue Response According to the Presence of Keratinized Mucosa. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e24–e28. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Schwarz, F.; Herrera, D.; McClain, P.; Figuero, E.; Molina, A.; Monje, A.; Montero, E.; Pascual, A.; Ramanauskaite, A.; et al. Importance of Keratinized Mucosa around Dental Implants: Consensus Report of Group 1 of the DGI/SEPA/Osteology Workshop. Clin. Oral Implants Res. 2022, 33 (Suppl. S23), 47–55. [Google Scholar] [CrossRef]
- Longoni, S.; Tinto, M.; Pacifico, C.; Sartori, M.; Andreano, A. Effect of Peri-Implant Keratinized Tissue Width on Tissue Health and Stability: Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2019, 34, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Maghaireh, H.; Grusovin, M.G.; Ziounas, I.; Worthington, H.V. Soft Tissue Management for Dental Implants: What Are the Most Effective Techniques? A Cochrane Systematic Review. Eur. J. Oral Implantol. 2012, 5, 221–238. [Google Scholar]
- Wu, Q.; Qu, Y.; Gong, P.; Wang, T.; Gong, T.; Man, Y. Evaluation of the Efficacy of Keratinized Mucosa Augmentation Techniques around Dental Implants: A Systematic Review. J. Prosthet. Dent. 2015, 113, 383–390. [Google Scholar] [CrossRef]
- Seibert, J.S. Reconstruction of Deformed, Partially Edentulous Ridges, Using Full Thickness Onlay Grafts. Part I. Technique and Wound Healing. Compend. Contin. Educ. Dent. 1983, 4, 437–453. [Google Scholar] [PubMed]
- Camargo, P.M.; Melnick, P.R.; Kenney, E.B. The Use of Free Gingival Grafts for Aesthetic Purposes. Periodontology 2000 2001, 27, 72–96. [Google Scholar] [CrossRef]
- Dorfman, H.S.; Kennedy, J.E.; Bird, W.C. Longitudinal Evaluation of Free Autogenous Gingival Grafts. A Four Year Report. J. Periodontol. 1982, 53, 349–352. [Google Scholar] [CrossRef]
- Keceli, H.G.; Sengun, D.; Berberoğlu, A.; Karabulut, E. Use of Platelet Gel with Connective Tissue Grafts for Root Coverage: A Randomized-Controlled Trial. J. Clin. Periodontol. 2008, 35, 255–262. [Google Scholar] [CrossRef]
- Cairo, F.; Pagliaro, U.; Nieri, M. Treatment of Gingival Recession with Coronally Advanced Flap Procedures: A Systematic Review. J. Clin. Periodontol. 2008, 35, 136–162. [Google Scholar] [CrossRef]
- Ioannou, A.L.; Kotsakis, G.A.; McHale, M.G.; Lareau, D.E.; Hinrichs, J.E.; Romanos, G.E. Soft Tissue Surgical Procedures for Optimizing Anterior Implant Esthetics. Int. J. Dent. 2015, 2015, 740764. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levine, R.A.; Huynh-Ba, G.; Cochran, D.L. Soft Tissue Augmentation Procedures for Mucogingival Defects in Esthetic Sites. Int. J. Oral Maxillofac. Implants 2014, 29, 155–185. [Google Scholar] [CrossRef]
- Sanz, M.; Lorenzo, R.; Aranda, J.J.; Martin, C.; Orsini, M. Clinical Evaluation of a New Collagen Matrix (Mucograft Prototype) to Enhance the Width of Keratinized Tissue in Patients with Fixed Prosthetic Restorations: A Randomized Prospective Clinical Trial. J. Clin. Periodontol. 2009, 36, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, R.; García, V.; Orsini, M.; Martin, C.; Sanz, M. Clinical Efficacy of a Xenogeneic Collagen Matrix in Augmenting Keratinized Mucosa around Implants: A Randomized Controlled Prospective Clinical Trial. Clin. Oral Implants Res. 2012, 23, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Puisys, A.; Zukauskas, S.; Kubilius, R.; Barbeck, M.; Razukevičius, D.; Linkevičiene, L.; Linkevičius, T. Clinical and Histologic Evaluations of Porcine-Derived Collagen Matrix Membrane Used for Vertical Soft Tissue Augmentation: A Case Series. Int. J. Periodontics Restor. Dent. 2019, 39, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Papi, P.; Pompa, G. The Use of a Novel Porcine Derived Acellular Dermal Matrix (Mucoderm) in Peri-Implant Soft Tissue Augmentation: Preliminary Results of a Prospective Pilot Cohort Study. Biomed. Res. Int. 2018, 2018, 6406051. [Google Scholar] [CrossRef]
- Zafiropoulos, G.-G.; Al-Asfour, A.A.; Abuzayeda, M.; Kačarević, Z.P.; Murray, C.A.; Trajkovski, B. Peri-Implant Mucosa Augmentation with an Acellular Collagen Matrix. Membranes 2021, 11, 698. [Google Scholar] [CrossRef]
- Horvath, A.; Molnar, B.; Gera, I.; Windisch, P. Comparison of Different Approaches Aimed at Increasing Peri-Implant Keratinised Mucosa. In Proceedings of the ITI World Symposium 2014, Geneva, Switzerland, 24–26 April 2014. [Google Scholar]
- Emanuel, E.J. Reconsidering the Declaration of Helsinki. Lancet 2013, 381, 1532–1533. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Moest, T.; Lutz, R.; Wehrhan, F.; Neukam, F.W.; Schlegel, K.A. Long-Term Outcomes after Vestibuloplasty with a Porcine Collagen Matrix (Mucograft ®) versus the Free Gingival Graft: A Comparative Prospective Clinical Trial. Clin. Oral Implants Res. 2016, 27, e125–e133. [Google Scholar] [CrossRef]
- Vignoletti, F.; Nunez, J.; Sanz, M. Soft Tissue Wound Healing at Teeth, Dental Implants and the Edentulous Ridge When Using Barrier Membranes, Growth and Differentiation Factors and Soft Tissue Substitutes. J. Clin. Periodontol. 2014, 41 (Suppl. S15), S23–S35. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Benner, M.; Fienitz, T.; Happe, A.; Kreppel, M.; Nickenig, H.-J.; Zöller, J.E. Biodegradation Pattern and Tissue Integration of Native and Cross-Linked Porcine Collagen Soft Tissue Augmentation Matrices—An Experimental Study in the Rat. Head. Face Med. 2014, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Pabst, A.M.; Ackermann, M.; Moergel, M.; Jung, J.; Kasaj, A. Biofunctionalization of Porcine-Derived Collagen Matrix Using Enamel Matrix Derivative and Platelet-Rich Fibrin: Influence on Mature Endothelial Cell Characteristics In Vitro. Clin. Oral Investig. 2018, 22, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.; Lehmann, K.-M.; Walter, C.; Krueger, M.; Stratul, S.-I.; Kasaj, A. Influence of Porcine-Derived Collagen Matrix on Endothelial Progenitor Cells: An in Vitro Study. Odontol. Soc. Nippon Dent. Univ. 2014, 104, 19–26. [Google Scholar] [CrossRef]
- Nandkeoliar, T.; Kumar, A.; Chungkham, S.; Singh, S.S. Estimation of Vestibular Depth: An Observational Cross-Sectional Study. J. Indian Soc. Periodontol. 2023, 27, 320–327. [Google Scholar] [CrossRef]
| Maxilla | Mandible | |
|---|---|---|
| Age | 55.92 ± 14.38 | 57.71 ± 11.2 |
| Male | 4 | 0 |
| Female | 8 | 12 |
| Premolar | 3 | 4 |
| Molar | 9 | 8 |
| Time | Mean | SD | p-Value |
|---|---|---|---|
| Initial | 0.42 | 0.47 | 0.00002 |
| 6 months | 3.17 | 1.21 | |
| Difference | 2.36 | 1.34 | |
| Initial | 0.42 | 0.47 | 0.00035 |
| 12 months | 2.36 | 1.34 | |
| Difference | 1.94 | 1.37 | |
| 6 months | 3.17 | 1.21 | 0.009 |
| 12 months | 2.36 | 1.34 | |
| Difference | 0.81 | 0.09 |
| Time | Mean | SD | p-Value |
|---|---|---|---|
| Initial | 0.29 | 0.45 | 0.010 |
| 6 months | 1.58 | 1.44 | |
| Difference | 1.29 | 0.70 | |
| Initial | 0.29 | 0.45 | 0.023 |
| 12 months | 1.08 | 1.07 | |
| Difference | 0.79 | 0.44 | |
| 6 months | 1.58 | 1.44 | 0.033 |
| 12 months | 1.08 | 1.07 | |
| Difference | 0.50 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, A.; Windisch, P.; Palkovics, D.; Li, X. Novel Technique to Reconstruct Peri-Implant Keratinised Mucosa Width Using Xenogeneic Dermal Matrix. Clinical Case Series. Dent. J. 2024, 12, 43. https://doi.org/10.3390/dj12030043
Horváth A, Windisch P, Palkovics D, Li X. Novel Technique to Reconstruct Peri-Implant Keratinised Mucosa Width Using Xenogeneic Dermal Matrix. Clinical Case Series. Dentistry Journal. 2024; 12(3):43. https://doi.org/10.3390/dj12030043
Chicago/Turabian StyleHorváth, Attila, Péter Windisch, Dániel Palkovics, and Xinda Li. 2024. "Novel Technique to Reconstruct Peri-Implant Keratinised Mucosa Width Using Xenogeneic Dermal Matrix. Clinical Case Series" Dentistry Journal 12, no. 3: 43. https://doi.org/10.3390/dj12030043
APA StyleHorváth, A., Windisch, P., Palkovics, D., & Li, X. (2024). Novel Technique to Reconstruct Peri-Implant Keratinised Mucosa Width Using Xenogeneic Dermal Matrix. Clinical Case Series. Dentistry Journal, 12(3), 43. https://doi.org/10.3390/dj12030043