Correlation between Periodontitis and Onset of Alzheimer’s Disease: A Literature Review
Abstract
1. Introduction
- -
- Stage 0: Asymptomatic, deterministic gene.
- -
- Stage 1: Asymptomatic, biomarker evidence only.
- -
- Stage 2: Transitional decline: mild detectable change, but minimal impact on daily function.
- -
- Stage 3: Cognitive impairment with early functional impact.
- -
- Stage 4: Dementia with mild functional impairment.
- -
- Stage 5: Dementia with moderate functional impairment.
- -
- Stage 6: Dementia with severe functional impairment.
2. Materials and Methods
3. Results
- -
- review 1: in favor of the research hypothesis.
- -
- review 2: in favor of the research hypothesis.
- -
- review 3: in favor of the research hypothesis.
- -
- review 4: in favor of the research hypothesis.
- -
- review 5: in favor of the research hypothesis.
- -
- review 6: partly in favor of the research hypothesis.
- -
- review 7: in favor of the research hypothesis.
- -
- review 8: in favor of the research hypothesis.
- -
- review 9: in favor of the research hypothesis.
- -
- review 10: in favor of the research hypothesis.
4. Discussion
4.1. Correlation Between Periodontitis and Alzheimer’s Disease
4.2. Correlation Between Periodontal Bacteria and Alzheimer’s Disease
4.3. Correlation with the Detection of Antibodies to Periodontal Bacteria and Alzheimer’s Disease
4.4. References to the Blood–Brain Barrier (BBB)
4.5. Correlation Between Periodontitis-Induced Pro-Inflammatory Cytokines and Alzheimer’s Disease
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindhe, J.; Lang, N.P. Parodontologia Clinica e Implantologia Orale, 7th ed.; Edi Ermes: Milan, Italy, 2016; pp. 208–211. [Google Scholar]
- Duda-Sobczak, A.; Zozulinska-Ziolkiewicz, D.; Wyganowska-Swiatkowska, M. Type 1 Diabetes and Periodontal Health. Clin. Ther. 2018, 40, 823–827. [Google Scholar] [CrossRef]
- Chapple, I.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S74–S84. [Google Scholar] [CrossRef]
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontol. 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Prado, M.M.; Figueiredo, N.; Pimenta, A.D.L.; Miranda, T.S.; Feres, M.; Figueiredo, L.C.; de Almeida, J.; Bueno-Silva, B. Recent updates on microbial biofilms in periodontitis: An analysis of in vitro biofilm models. Periodontitis 2022, 1373, 159–174. [Google Scholar]
- Yucel-Lindberg, T.; Båge, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dye, B.A. The Global Burden of Oral Disease: Research and Public Health Significance. J. Dent. Res. 2017, 96, 361–363. [Google Scholar] [CrossRef]
- Janakiram, C.; Dye, B.A. A public health approach for prevention of periodontal disease. Periodontol. 2000 2020, 84, 202–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcenes, W.; Kassebaum, N.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Mensah, G.A.; Krish, V.; Glenn, S.D.; Miller-Petrie, M.K.; Lopez, A.D.; Murray, C.J.L. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Jin, L.J.; Lamster, I.B.; Greenspan, J.S.; Pitts, N.B.; Scully, C.; Warnakulasuriya, S. Global burden of oral diseases: Emerging concepts, management and interplay with systemic health. Oral Dis. 2016, 22, 609–619. [Google Scholar] [CrossRef]
- Raspini, M.; Cavalcanti, R.; Clementini, M.; Crea, A.; Di Stefano, M.; Fratini, A.; Karaboue, M.; Lacasella, G.; Landi, L.; Littarru, C.; et al. Periodontitis and Italians (2016–2020): Need for clinical guidelines to perform effective therapy. Dent. Cadmos 2021, 89, 346–356. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontol. 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Giorgio, V.; Marcello, C.; Pini, P.G.P. Parodontologia Clinica; Quintessenza: Milan, Italy, 2011; p. 134. [Google Scholar]
- Aja, E.; Mangar, M.; Fletcher, H.M.; Mishra, A. Filifactor alocis: Recent Insights and Advances. J. Dent. Res. 2021, 100, 790–797. [Google Scholar] [CrossRef]
- Binshabaib, M.; ALHarthi, S.S.; Salehpoor, D.; Michelogiannakis, D.; Javed, F. Contribution of herpesviruses in the progression of periodontal and peri-implant diseases in systemically healthy individuals. Rev. Med. Virol. 2018, 28, e1996. [Google Scholar] [CrossRef]
- Slots, J.; Contreras, A. Herpesviruses: A unifying causative factor in periodontitis? Oral Microbiol. Immunol. 2000, 15, 277–280. [Google Scholar] [CrossRef]
- Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde Allgemeine Zeitschrift fur Psychiatrie und Psychisch-gerichtliche Medizin. Clin. Anat. 1907, 64, 146–168. [Google Scholar]
- Alzheimer, A.; Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An English translation of Alzheimer’s 1907 paper, Uber eine eigenartige Erkankung der Hirnrinde. Clin Anat. 1995, 8, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.A.; Syty, M.D.; Wu, K.; Ge, S. Adult hippocampal neurogenesis and its impairment in Alzheimer’s disease. Zool. Res. 2022, 43, 481–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Alessandro, G.; Costi, T.; Alkhamis, N.; Bagattoni, S.; Sadotti, A.; Piana, G. Oral Health Status in Alzheimer’s Disease Patients: A Descriptive Study in an Italian Population. J. Contemp. Dent. Pract. 2018, 19, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Delwel, S.; Binnekade, T.T.; Perez, R.S.G.M.; Hertogh, C.M.P.M.; Scherder, E.J.A.; Lobbezoo, F. Oral hygiene and oral health in older people with dementia: A comprehensive review with focus on oral soft tissues. Clin. Oral Investig. 2018, 22, 93–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kane, S.F. The effects of oral health on systemic health. Gen. Dent. 2017, 65, 30–34. [Google Scholar] [PubMed]
- Holmer, J.; Eriksdotter, M.; Schultzberg, M.; Pussinen, P.J.; Buhlin, K. Association between periodontitis and risk of Alzheimer’s disease, mild cognitive impairment and subjective cognitive decline: A case-control study. J. Clin. Periodontol. 2018, 45, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Wiste, H.J.; Botha, H.; Weigand, S.D.; Therneau, T.M.; Knopman, D.S.; Graff-Radford, J.; Jones, D.T.; Ferman, T.J.; Boeve, B.F.; et al. The bivariate distribution of amyloid-beta and tau: Relationship with established neurocognitive clinical syndromes. Brain 2019, 142, 3230–3242. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Chételat, G.; Dickson, D.; Fagan, A.M.; Frisoni, G.B.; Jagust, W.; Mormino, E.C.; Petersen, R.C.; Sperling, R.A.; et al. Suspected non-Alzheimer disease pathophysiology—Concept and controversy. Nat. Rev. Neurol. 2016, 12, 117–124. [Google Scholar] [CrossRef]
- Said-Sadier, N.; Sayegh, B.; Farah, R.; Abbas, L.A.; Dweik, R.; Tang, N.; Ojcius, D.M. Association between Periodontal Disease and Cognitive Impairment in Adults. Int. J. Environ. Res. Public Health 2023, 20, 4707. [Google Scholar] [CrossRef]
- Parra-Torres, V.; Melgar-Rodríguez, S.; Muñoz-Manríquez, C.; Sanhueza, B.; Cafferata, E.A.; Paula-Lima, A.C.; Díaz-Zúñiga, J. Periodontal bacteria in the brain-Implication for Alzheimer’s disease: A systematic review. Oral Dis. 2023, 29, 21–28. [Google Scholar] [CrossRef]
- Mao, S.; Huang, C.P.; Lan, H.; Lau, H.G.; Chiang, C.P.; Chen, Y.W. Association of periodontitis and oral microbiomes with Alzheimer’s disease: A narrative systematic review. J. Dent. Sci. 2022, 17, 1762–1779. [Google Scholar] [CrossRef]
- Pisani, F.; Pisani, V.; Arcangeli, F.; Harding, A.; Singhrao, S.K. The Mechanistic Pathways of Periodontal Pathogens Entering the Brain: The Potential Role of Treponema denticola in Tracing Alzheimer’s Disease Pathology. Int. J. Environ. Res. Public Health 2022, 19, 9386. [Google Scholar] [CrossRef]
- Kaliamoorthy, S.; Nagarajan, M.; Sethuraman, V.; Jayavel, K.; Lakshmanan, V.; Palla, S. Association of Alzheimer’s disease and periodontitis—A systematic review and meta-analysis of evidence from observational studies. Med. Pharm. Rep. 2022, 95, 144–151. [Google Scholar] [CrossRef]
- Elwishahy, A.; Antia, K.; Bhusari, S.; Ilechukè, N.C.; Horstick, O.; Winkler, V. Porphyromonas gingivalis as a Risk Factor to Alzheimer’s Disease: A Systematic Review. J. Alzheimers Dis. Rep. 2021, 5, 721–732. [Google Scholar] [CrossRef]
- Borsa, L.; Dubois, M.; Sacco, G.; Lupi, L. Analysis the Link between Periodontal Diseases and Alzheimer’s Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 9312. [Google Scholar] [CrossRef]
- Lorenzi, C.; Bianchi, N.; Pinto, A.; Mazzetti, V.; Arcuri, C. The role of periodontal bacteria, Porphyromonas Gingivalis, in Alzheimer’s disease pathogenesis and aggravation: A review. J. Biol. Regul. Homeost. Agents 2021, 35, 37–45. [Google Scholar]
- Costa, M.J.F.; de Araújo, I.D.T.; da Rocha Alves, L.; da Silva, R.L.; Dos Santos Calderon, P.; Borges, B.C.D.; de Aquino Martins, A.R.L.; de Vasconcelos Gurgel, B.C.; Lins, R.D.A.U. Relationship of Porphyromonas gingivalis and Alzheimer’s disease: A systematic review of pre-clinical studies. Clin. Oral Investig. 2021, 25, 797–806. [Google Scholar] [CrossRef]
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Mastrangelo, F.; Russo, L.L.; Muzio, L.L. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2020, 9, 495. [Google Scholar] [CrossRef]
- Villar, A.; Paladini, S.; Cossatis, J. Periodontal Disease and Alzheimer’s: Insights from a Systematic Literature Network Analysis. J. Prev. Alzheimers Dis. 2024, 11, 1148–1165. [Google Scholar] [CrossRef]
- Wu, D.T.; Cho, Y.W.; Spalti, M.D.; Bishara, M.; Nguyen, T.T. The link between periodontitis and Alzheimer’s disease—Emerging clinical evidence. Dent. Rev. 2023, 3, 100062. [Google Scholar] [CrossRef]
- Lundergan, W.; Parthasarathy, K.; Knight, N. Periodontitis and Alzheimer’s Disease: Is There a Connection? Oral 2024, 4, 61–73. [Google Scholar] [CrossRef]

| N° Article | Authors, Title, Journal, Year of Publication | Objective | Studies and Databases | Conclusions |
|---|---|---|---|---|
| 1 | Najwane Said Sadier, Batoul Sayegh, Raymond Farah, Linda Abou Abbas, Rania Dweik, Norina Tang, David M Ojcius, Association between periodontal disease and cognitive impairment in adults [31]. | Systematic review with the purpose of evaluating whether there is a greater risk of cognitive deterioration among adult patients with periodontitis with age equal to or greater than 18 years in comparison to adults without periodontitis. | 11 observational studies published by September 2021 on PubMed, Web of Science, and CINAHL. | Patients exposed to chronic periodontitis for at least eight years show an increase risk of developing cognitive decline or AD; alveolar bone loss increases 2.1 times the risk of developing cognitive impairment. However, some responsible mechanisms are still unclear and deserve further investigation. |
| 2 | Valeria Parra-Torres, Samanta Melgar-Rodríguez, Constanza Muñoz-Manríquez, Benjamín Sanhueza, Emilio ACafferata, Andrea C Paula-Lima, Jaime Díaz-Zúñiga, Periodontal bacteria in the brain-Implication for Alzheimer’s disease: A systematic review [32]. | Systematic review with the aim of evaluating the presence of periodontal bacteria in the brain and their role in the debut and progression of AD in humans and animals. | 23 observational studies and experiments published between 2002 and 2020 on Medline, Latindex, SciELO, and Cochrane Library databases. | Infection by oral pathogens in animals was related to the development of neuropathological characteristics of AD and the detection of bacteria in the brain. In patients with AD, oral bacteria were detected in brain tissues at pro-cytokines inflammatory increasing levels. Bacteria in the brain are related to pathological characteristics of AD. However, it was discovered that oral bacteria can be detected in the brain even in the absence of periodontitis, therefore the bacterial virulence and the host susceptibility will influence the potential neuroinflammatory response. |
| 3 | Samantha Mao, Chen-Pang Huang, Hsin Lan, Hing-Ger Lau, Chun-Pin Chiang, Yi-Wen Chen, Association of periodontitis and oral microbiomes with Alzheimer’s disease: A narrative systematic review [33]. | Systematic review with the aim of evaluating the correlation between oral microbiome and development of AD. | 26 observational studies published before November 2021 on Pubmed, Embase, and Google Scholar. | Periodontal pathogens play a role in the pathogenesis of AD; however, it would be appropriate to standardize the operating protocol and the sampling site to obtain clearer results. Further studies are also needed to clarify whether periodontal therapies have positive feedback on the prevention and cure of AD. |
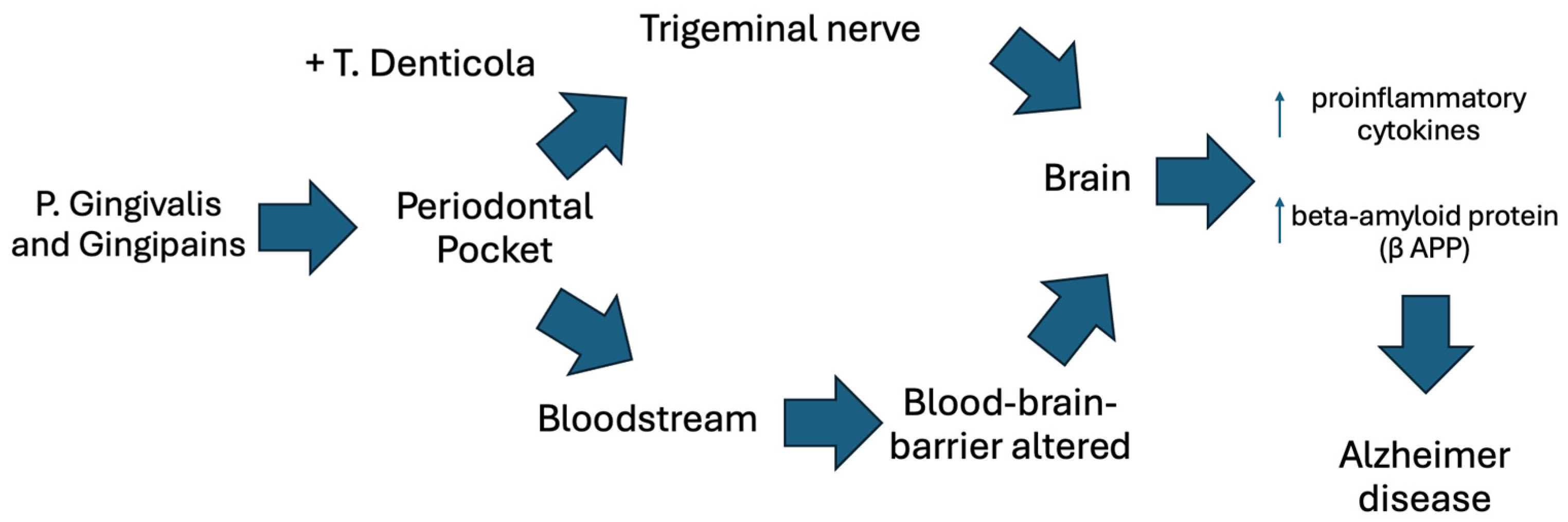
| 4 | Flavio Pisani, Valerio Pisani, Francesca Arcangeli, Alice Harding, Simarjit Kaur Singhrao, The mechanism pathways of periodontal pathogenesis entering the brain: the potential role of Treponema denticola in tracing Alzheimer’s disease pathology [34]. | Systematic review with the aim of evaluating the methods of entry of periodontal pathogens to the brain with a focus on T. Denticola. | 99 observational studies published before July 2022 on Pubmed and Google Scholar. | The spread of T. Denticola in the blood circulatory system causes neuro-inflammation of the Trigeminal nerve and, where present, increases the blood–brain barrier permeability favoring the entry of other pathogens into the brain, including P. Gingivalis, implicated directly into the lesion formation typical of AD. Furthermore, it highlights the importance of oral hygiene for containment of pathogenic bacteria present. |
| 5 | Sriram Kaliamoorthy, Mahendirakumar Nagarajan, Vijayparthiban Sethuraman, Kavitha Jayavel, Vijayalakshmi Lakshmanan, Santosh Palla, Association of Alzheimer’s disease and periodontitis-a systematic review and meta-analysis of evidence from observational studies [35]. | Systematic review and meta-analysis with the aim of investigating if adult individuals exposed to periodontitis or periodontal pathogens have a greater probability of developing AD. | 6 observational studies published between January 2010 and March 2020 on PubMed, Google Scholar, Web of Science, EMBASE, and Scopus. | Patients with periodontitis present a greater probability of developing AD compared to patients without periodontitis. It highlights the importance of oral hygiene in patients with AD. |
| 6 | Abdelrahman Elwishahy, Khatia Antia, Sneha Bhusari, Nkorika Chiamaka Ilechukwu, Olaf Horstick, Volker Winkler, Porphyromonas Gingivalis as a Risk Factor to Alzheimer’s Disease: A Systematic Review [36]. | Systematic review with the aim of identifying if exposure to P. Gingivalis and its virulence factors increase the risk of the onset of AD. | 6 observational studies published before the 31st August 2020 on Cochrane Library, Google Scholar, Ovid, PubMed, Web of Science, and WHOLIS. | The results have not shown a clear association between P. Gingivalis and AD; however, the majority of the studies taken into consideration suggest that the bacterium P. Gingivalis, through its virulence factors, is important in the process of systematic inflammation. Furthermore, this study revealed heterogeneity in the methodologies of measurement. |
| 7 | Leslie Borsa, Margaux Dubois, Guillaume Sacco, Laurence Lupi, Analysis the link between periodontal disease and Alzheimer’s disease: a systematic review [37]. | Systematic review and meta-analysis with the primary objective to evaluate the correlation between periodontitis and AD in patients of equal age or over 65 years old and the secondary objective to determine the presence of specific bacterial pathogens involved in periodontal disease. | 5 observational studies published between 2010 and June 21, 2021 on PubMed, Cochrane Library, and EMBASE. | The selected studies have found a relationship between periodontitis and AD, denoting that exposure to chronic periodontitis for at least 10 years increases the risk of occurrence of AD by 1.7 times; in patients with AD it was found that periodontopathogenic bacteria associated with a higher risk, high incidence, or mortality due to AD including P. Gingivalis, Campylobacter Rectus, and Fusobactrium Nucleatum. Further studies are necessary to better understand the etiology and the mechanisms of this interaction. A better understanding will permit the improvement of effective prevention measures and even care. The treatment of pockets, therefore, could represent a strategy for managing AD. |
| 8 | C. Lorenzi, N. Bianchi, A. Pinto, V. Mazzetti, C. Arcuri, Association of Alzheimer’s The role of periodontal bacteria, Porphyromonas gingivalis, in Alzheimer’s disease pathogenesis and aggravation: a review [38]. | Systematic review with the purpose of establishing the role of periodontal bacteria, especially in P. Gingivalis, in the pathogenesis of AD. | 9 observational studies published between 2016 and May 2021 on PubMed, Cochrane Library, and Web of Science. | The correlation between periodontal bacteria and AD is confirmed; however, the pathogenetic mechanisms are still not completely clear. In the studies, the presence of P. Gingivalis was detected in the brain and the CSF of AD patients; the patients affected by chronic periodontitis present an increase in concentration of IL-6 e TNF-α. A study states that ten-year exposure to periodontitis increases the risk of developing AD by 1.7 times. |
| 9 | Moan Jéfter Fernandes Costa, Isabela Dantas Torres de Araújo, Luana da Rocha Alves, Romerito Lins da Silva, Patricia Dos Santos Calderon, Boniek Castillo Dutra Borges, Ana Rafaela Luz de Aquino Martins, Bruno Cesar de Vasconcelos Gurgel, Ruthineia Diogenes Alves Uchoa Lins, Relationship of Porphyromonas gingivalis and Alzheimer’s disease: a systematic review of pre-clinical studies [39]. | Systematic review with the purpose of evaluating whether the animals infected with P. Gingivalis are more affected by AD compared to healthy ones. | 9 observational studies and published experiments between 2015 and 2019 on PubMed, LILACS, SciELO, ScienceDirect, Web of Science, Cochrane, and SCOPUS. | In animals, a correlation is amply demonstrated between AD and infection with Pg-LPS or P. Gingivalis, also mediated from the gingipains, which activates the waterfall complement, increases the production of Aβ and enhances the expression of pro-inflammatory cytokines, causing cerebral inflammation, neuroinflammation, and neurodegeneration, promoting cognitive deterioration. The analyzed studies indicate that periodontitis also has a harmful effect on the development and progression of AD. |
| 10 | Mario Dioguardi, Vito Crincoli, Luigi Laino, Mario Alovisi, Diego Sovereto, Filiberto Mastrangelo, Lucio Lo Russo, Lorenzo Lo Muzio, The role of periodontitis and periodontal bacteria in the onset and progression of Alzheimer’s Disease [40]. | Systematic review with the purpose of evaluating the correlation between periodontal bacteria and AD. | 15 observational studies published between 1989 and 2019 on PubMed and SCOPUS. | The analysis of the literature of scientific evidence shows that periodontitis can contribute to inflammation of the peripheral environment through the introduction of periodontal bacteria and pro-inflammatory cytokines favoring the onset of AD; however, further careful investigations on periodontal pathogens are necessary. |
| AD Patients | Healthy Patients |
|---|---|
| Slackia exigua | Actinomyces |
| Lachnospiraceae | Rothia |
| Prevotella oulorum | |
| Moraxella | |
| Leptotrichia | |
| Sphaerochaeta | |
| F. Nucleatum |
| Research Gap | Future Research Ideas |
|---|---|
| Absence of univocal results | To establish international guidelines on diagnosis and methods of analysis |
| Poor statistical analysis to better demonstrate the correlations between AD and PD | Experimental studies with the aim of establishing not only the bacterial species and inflammatory molecules involved in this process but also their quantities necessary for the initiation of cognitive impairment |
| Data not sufficient to relate this correlation to the age and sex of the patients | Experimental studies with the aim of establishing the impact of the therapeutic countermeasures taken by professionals in our field to slow or stop the progression of the pathology |
| Different diagnostic methods, analytical methods, and definition of periodontitis did not permit a very detailed comparison of the studies because they were not homologous | Further studies with a unique definition of periodontitis, following the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions are needed, to control for confounding variables |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbarisi, A.; Visconti, V.; Lauritano, D.; Cremonini, F.; Caccianiga, G.; Ceraulo, S. Correlation between Periodontitis and Onset of Alzheimer’s Disease: A Literature Review. Dent. J. 2024, 12, 331. https://doi.org/10.3390/dj12100331
Barbarisi A, Visconti V, Lauritano D, Cremonini F, Caccianiga G, Ceraulo S. Correlation between Periodontitis and Onset of Alzheimer’s Disease: A Literature Review. Dentistry Journal. 2024; 12(10):331. https://doi.org/10.3390/dj12100331
Chicago/Turabian StyleBarbarisi, Antonio, Valeria Visconti, Dorina Lauritano, Francesca Cremonini, Gianluigi Caccianiga, and Saverio Ceraulo. 2024. "Correlation between Periodontitis and Onset of Alzheimer’s Disease: A Literature Review" Dentistry Journal 12, no. 10: 331. https://doi.org/10.3390/dj12100331
APA StyleBarbarisi, A., Visconti, V., Lauritano, D., Cremonini, F., Caccianiga, G., & Ceraulo, S. (2024). Correlation between Periodontitis and Onset of Alzheimer’s Disease: A Literature Review. Dentistry Journal, 12(10), 331. https://doi.org/10.3390/dj12100331









