In Vitro Models Used in Cariology Mineralisation Research—A Review of the Literature
Abstract
1. Introduction
2. Methods
3. Results and Discussion
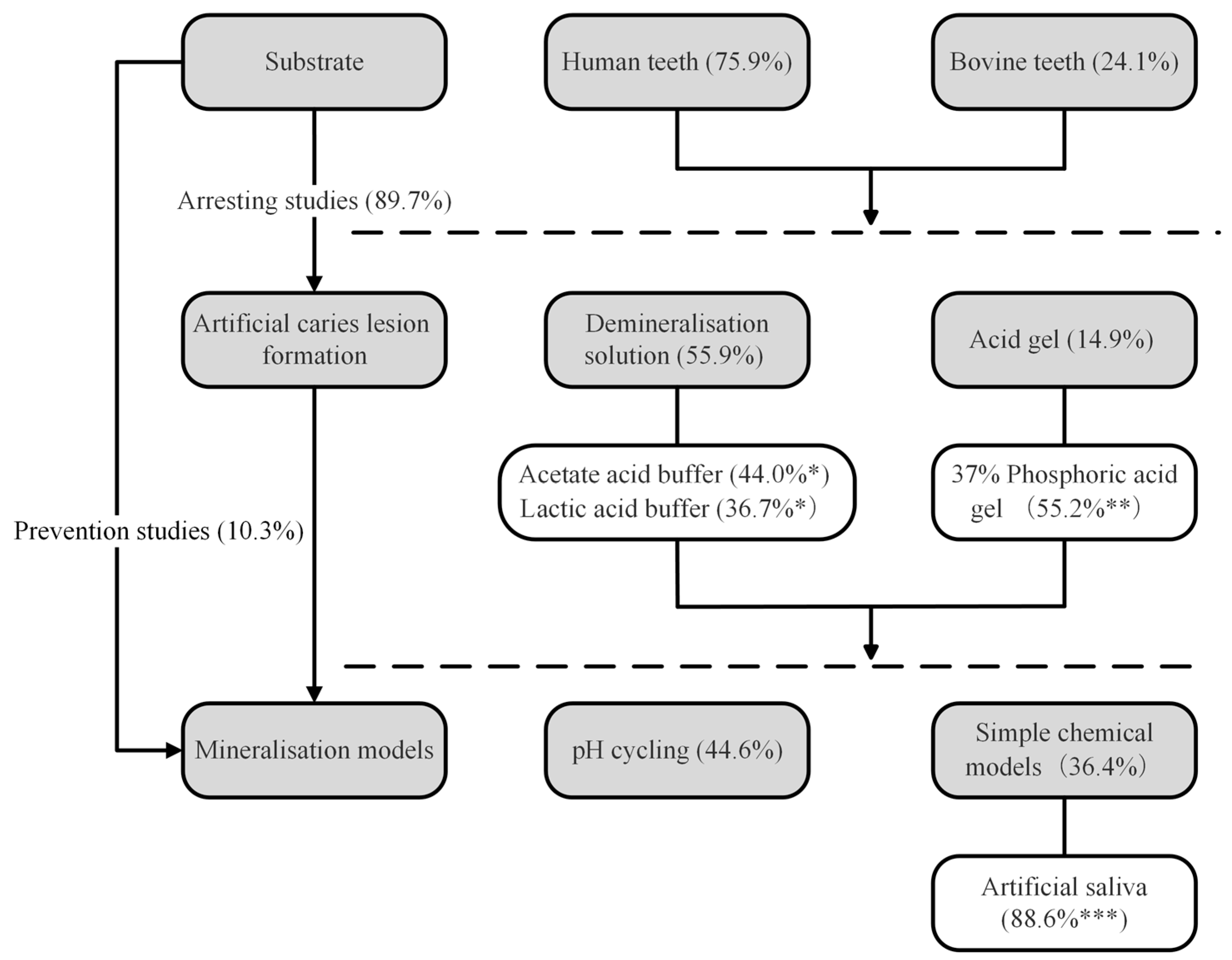
3.1. Substrates
3.1.1. Human Teeth
3.1.2. Bovine Teeth
3.1.3. Specimen Storage, Inclusion Criteria, and Preparation
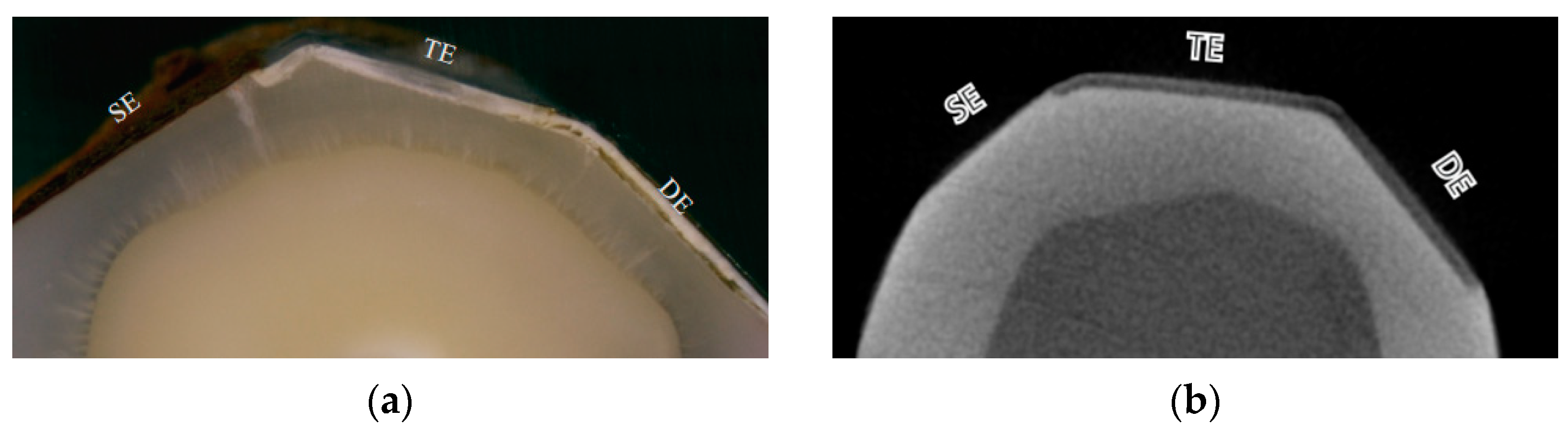
3.1.4. Specimen Preparation (Surface Preparation and Internal Controls)
3.2. Artificial Caries Lesion Formation
3.2.1. Acid Solution
3.2.2. Acid Gel
3.3. Mineralisation Models
3.3.1. The pH Cycling Model
3.3.2. Simple Chemical Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fejerskov, O.; Nyvad, B.; Kidd, E. Dental Caries: The Disease and Its Clinical Management; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Degli Esposti, L.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Iafisco, M. Characterization of a Toothpaste Containing Bioactive Hydroxyapatites and In Vitro Evaluation of Its Efficacy to Remineralize Enamel and to Occlude Dentinal Tubules. Materials 2020, 13, 2928. [Google Scholar] [CrossRef] [PubMed]
- Bahrololoomi, Z.; Zarebidoki, F.; Mostafalu, N. The effect of different re-mineralizing agents and diode laser irradiation on the microhardness of primary molar enamel: An in vitro study. Laser Ther. 2019, 28, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Arifa, M.K.; Ephraim, R.; Rajamani, T. Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int. J. Clin. Pediatr. Dent. 2019, 12, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Schuthof, J.; Christoffersen, J. Inhibition of enamel demineralization by albumin in vitro. Caries Res. 1986, 20, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.R.; Pessan, J.P.; Honório, H.M.; Ten Cate, J.M. Mechanisms of action of fluoride for caries control. Fluoride Oral Environ. 2011, 22, 97–114. [Google Scholar]
- Lata, S.; Varghese, N.O.; Varughese, J.M. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation. J. Conserv. Dent. 2010, 13, 42–46. [Google Scholar] [CrossRef]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.; Chu, C.H. A Review of the Common Models Used in Mechanistic Studies on Demineralization-Remineralization for Cariology Research. Dent. J. 2017, 5, 20. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Townsend, G.; Bockmann, M.; Hughes, T.; Brook, A. Genetic, environmental and epigenetic influences on variation in human tooth number, size and shape. Odontology 2012, 100, 1–9. [Google Scholar] [CrossRef]
- Vieira, A.P.G.F.; Hanocock, R.; Eggertsson, H.; Everett, E.T.; Grynpas, M.D. Tooth Quality in Dental Fluorosis. Calcif. Tissue Int. 2005, 76, 17–25. [Google Scholar] [CrossRef]
- Carvalho, T.S.; Lussi, A. Age-related morphological, histological and functional changes in teeth. J. Oral Rehabil. 2017, 44, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Wahba, N.; Schwendicke, F.; Kamel, M.A.; Allam, G.; Kabil, N.; Elhennawy, K. Preventing and Arresting Primary Tooth Enamel Lesions Using Self- Assembling Peptide P(11)-4 In Vitro. J. Int. Soc. Prev. Community Dent. 2022, 12, 58–70. [Google Scholar] [CrossRef]
- Mahfouz Omer, S.M.; El-Sherbiny, R.H.; El-Desouky, S.S. Effect of N-Acetylcysteine on initial Carious Enamel Lesions in primary teeth: An In-vitro study. BMC Oral Health 2023, 23, 520. [Google Scholar] [CrossRef] [PubMed]
- De Menezes Oliveira, M.A.; Torres, C.P.; Gomes-Silva, J.M.; Chinelatti, M.A.; De Menezes, F.C.; Palma-Dibb, R.G.; Borsatto, M.C. Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc. Res. Tech. 2010, 73, 572–577. [Google Scholar] [CrossRef]
- Teruel Jde, D.; Alcolea, A.; Hernández, A.; Ruiz, A.J. Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch. Oral Biol. 2015, 60, 768–775. [Google Scholar] [CrossRef]
- Olek, A.; Klimek, L.; Bołtacz-Rzepkowska, E. Comparative scanning electron microscope analysis of the enamel of permanent human, bovine and porcine teeth. J. Vet. Sci. 2020, 21, e83. [Google Scholar] [CrossRef] [PubMed]
- Gwinnett, A.J.; Buonocore, M.G.; Sheykholeslam, Z. Effect of fluoride on etched human and bovine tooth enamel surfaces as demonstrated by scanning electron microscopy. Arch. Oral Biol. 1972, 17, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Yassen, G.H.; Platt, J.A.; Hara, A.T. Bovine teeth as substitute for human teeth in dental research: A review of literature. J. Oral Sci. 2011, 53, 273–282. [Google Scholar] [CrossRef]
- Arends, J.; Jongebloed, W.L. Crystallites dimensions of enamel. J. Biol. Buccale 1978, 6, 161–171. [Google Scholar]
- Ayoub, H.M.; Gregory, R.L.; Tang, Q.; Lippert, F. Comparison of human and bovine enamel in a microbial caries model at different biofilm maturations. J. Dent. 2020, 96, 103328. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Higham, S.M.; Edgar, W.M. Factors influencing the development of dental erosion in vitro: Enamel type, temperature and exposure time. J. Oral Rehabil. 1999, 26, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Reed, R.; Gorski, J.P.; Wang, Y.; Walker, M.P. The Distribution of Carbonate in Enamel and its Correlation with Structure and Mechanical Properties. J. Mater. Sci. 2012, 47, 8035–8043. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.Y.W.; Lim, S.L.; Loi, S.T.Y.; Mei, M.L.; Li, K.C.; Aziz, S.; Ekambaram, M. A comparative study of two chemical models for creating subsurface caries lesions on aprismatic and prismatic enamel. J. Oral Sci. 2023, 65, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Aydın, B.; Pamir, T.; Baltaci, A.; Orman, M.N.; Turk, T. Effect of storage solutions on microhardness of crown enamel and dentin. Eur. J. Dent. 2015, 9, 262–266. [Google Scholar] [CrossRef]
- Secilmis, A.; Dilber, E.; Gokmen, F.; Ozturk, N.; Telatar, T. Effects of storage solutions on mineral contents of dentin. J. Dent. Sci. 2011, 6, 189–194. [Google Scholar] [CrossRef]
- Ten Cate, J.M.; Duijsters, P.P. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982, 16, 201–210. [Google Scholar] [CrossRef]
- Pashley, D.H. Dynamics of the Pulpo-Dentin Complex. Crit. Rev. Oral Biol. Med. 1996, 7, 104–133. [Google Scholar] [CrossRef]
- Love, R.M.; Jenkinson, H.F. Invasion of dentinal tubules by oral bacteria. Crit. Rev. Oral Biol. Med. 2002, 13, 171–183. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.-P.; Zhu, J.; Yang, J.; Zhu, H.-S. Impact of Dentinal Tubule Orientation on Dentin Bond Strength. Curr. Med. Sci. 2018, 38, 721–726. [Google Scholar] [CrossRef]
- Nath, S.J.C.; Fu, Y.; Li, K.C.; Loho, T.; Loch, C.; Ekambaram, M. A Comparison of the Enamel Remineralisation Potential of Self-Assembling Peptides. Int. Dent. J. 2024, 74, 187–194. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Karnowakul, J.; Punyanirun, K.; Jirakran, K.; Thanyasrisung, P.; Techatharatip, O.; Pornprasertsuk-Damrongsri, S.; Trairatvorakul, C. Enhanced effectiveness of silver diamine fluoride application with light curing on natural dentin carious lesions: An in vitro study. Odontology 2023, 111, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Kongsomjit, M.; Punyanirun, K.; Tasachan, W.; Hamba, H.; Tagami, J.; Trairatvorakul, C.; Thanyasrisung, P. Material of choice for non-invasive treatment of dentin caries: An in vitro study using natural carious lesions. Int. J. Dent. Hyg. 2024, 22, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Orilisi, G.; Vitiello, F.; Notarstefano, V.; Furlani, M.; Riberti, N.; Monterubbianesi, R.; Bellezze, T.; Campus, G.; Carrouel, F.; Orsini, G.; et al. Multidisciplinary evaluation of the remineralization potential of three fluoride-based toothpastes on natural white spot lesions. Clin. Oral Investig. 2023, 27, 7451–7462. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.C.; Burwell, A.K.; Saeki, K.; Fernandez-Martinez, A.; Pugach, M.K.; Nonomura, G.; Habelitz, S.; Ho, S.P.; Rapozo-Hilo, M.; Featherstone, J.D.; et al. Distinct decalcification process of dentin by different cariogenic organic acids: Kinetics, ultrastructure and mechanical properties. Arch. Oral Biol. 2016, 63, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Eggers, K.; Meyer-Lueckel, H.; Dörfer, C.; Kovalev, A.; Gorb, S.; Paris, S. In vitro Induction of residual caries lesions in dentin: Comparative mineral loss and nano-hardness analysis. Caries Res. 2015, 49, 259–265. [Google Scholar] [CrossRef]
- Rajendran, R.; Hussain, M.S.; Sandhya, R.; Thomas, A.J.; Ameena, M.; Saleem, S. Comparative evaluation of remineralisation potential of bioactive glass, casein phosphopeptide-amorphous calcium phosphate and novel strontium-doped nanohydroxyapatite paste: An In-Vitro study. Indian J. Dent. Res. 2022, 33, 94–99. [Google Scholar] [CrossRef]
- Darshan, H.E.; Shashikiran, N.D. The effect of McInnes solution on enamel and the effect of Tooth mousse on bleached enamel: An in vitro study. J. Conserv. Dent. 2008, 11, 86–91. [Google Scholar] [CrossRef]
- Rajendran, R.; Nair, K.R.; Sandhya, R.; Ashik, P.M.; Veedu, R.P.; Saleem, S. Evaluation of remineralization potential and cytotoxicity of a novel strontium-doped nanohydroxyapatite paste: An in vitro study. J. Conserv. Dent. 2020, 23, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Kamath, K.A.; Nasim, I.; Rajeshkumar, S. Evaluation of the re-mineralization capacity of a gold nanoparticle-based dental varnish: An in vitro study. J. Conserv. Dent. 2020, 23, 390–394. [Google Scholar] [CrossRef]
- Ngoc, V.T.N.; Coulton, K.M.; Tra, N.T.; My, N.H.; Huong, P.T.Q.; Son, T.M.; Anh, L.Q.; Thuy, L.Q.; Anh, T.T.; Dinh, T.C.; et al. HMU Fluorinze Mouthwash Enhances Enamel Remineralization: An In Vitro Study. Open Access Maced. J. Med. Sci. 2019, 7, 4319–4323. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.; Singh, N.; Shaila; Thomas, A.M.; Jairath, R. A comparative evaluation of penetration depth and surface microhardness of Resin Infiltrant, CPP-ACPF and Novamin on enamel demineralization after banding: An in vitro study. Biomater. Investig. Dent. 2021, 8, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.H.C.; Oliveira, M.R.C.; Oliveira, L.H.C.; Sfalcin, R.A.; Pinto, M.M.; Rosa, E.P.; Melo Deana, A.; Horliana, A.; César, P.F.; Bussadori, S.K. Evaluation of Different Dentifrice Compositions for Increasing the Hardness of Demineralized Enamel: An in Vitro Study. Dent. J. 2019, 7, 14. [Google Scholar] [CrossRef]
- Da Freiria, A.C.B.; Ortiz, M.I.G.; de Sobral, D.F.S.; Aguiar, F.H.B.; Lima, D. Nano-hydroxyapatite-induced remineralization of artificial white spot lesions after bleaching treatment with 10% carbamide peroxide. J. Esthet. Restor. Dent. 2022, 34, 1290–1299. [Google Scholar] [CrossRef]
- Nunes, G.P.; Danelon, M.; Pessan, J.P.; Capalbo, L.C.; Junior, N.A.N.; Matos, A.A.; Souza, J.A.S.; Buzalaf, M.A.R.; Delbem, A.C.B. Fluoride and trimetaphosphate association as a novel approach for remineralization and antiproteolytic activity in dentin tissue. Arch. Oral Biol. 2022, 142, 105508. [Google Scholar] [CrossRef] [PubMed]
- Borompiyasawat, P.; Putraphan, B.; Luangworakhun, S.; Sukarawan, W.; Techatharatip, O. Chlorhexidine gluconate enhances the remineralization effect of high viscosity glass ionomer cement on dentin carious lesions in vitro. BMC Oral Health 2022, 22, 60. [Google Scholar] [CrossRef]
- Marquezan, M.; Corrêa, F.N.; Sanabe, M.E.; Rodrigues Filho, L.E.; Hebling, J.; Guedes-Pinto, A.C.; Mendes, F.M. Artificial methods of dentine caries induction: A hardness and morphological comparative study. Arch. Oral Biol. 2009, 54, 1111–1117. [Google Scholar] [CrossRef]
- Dohan, Z.; Friedlander, L.T.; Cooper, P.R.; Li, K.C.; Ratnayake, J.T.; Mei, M.L. In Vitro Models Used in the Formation of Root Caries Lesions-A Review of the Literature. Dent. J. 2023, 11, 269. [Google Scholar] [CrossRef]
- Ngoc, C.H.; Manh, D.T.; Le, H. An Experimental and Clinically Controlled Study of the Prevention of Dental Caries Using 1.23% Fluoride Gel in Elderly Patients. J. Int. Soc. Prev. Community Dent. 2021, 11, 661–670. [Google Scholar] [CrossRef]
- Zalite, V.; Lungevics, J.; Vecstaudza, J.; Stipniece, L.; Locs, J. Nanosized calcium deficient hydroxyapatites for tooth enamel protection. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1354–1367. [Google Scholar] [CrossRef]
- Valizadeh, S.; Rahimi Khub, M.; Chiniforush, N.; Kharazifard, M.J.; Hashemikamangar, S.S. Effect of Laser Irradiance and Fluoride Varnish on Demineralization Around Dental Composite Restorations. J. Lasers Med. Sci. 2020, 11, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, D.; Zhou, Z.; Sun, Y.; Pan, X.; Liu, W.; Chu, C.H.; Zhang, L.; Hannig, M.; Fu, B. Polyelectrolyte-Cation Complexes Using PAsp-Sr Complexes Induce Biomimetic Mineralization with Antibacterial Ability. Adv. Healthc. Mater. 2024, 13, e2303002. [Google Scholar] [CrossRef] [PubMed]
- Heshmat, H.; Kazemi, H.; Hoorizad Ganjkar, M.; Chaboki, F.; Shokri, M.; Kharazifard, M.J. Effect of Two Remineralizing Agents on Dentin Microhardness of Non-Caries Lesions. J. Dent. 2023, 24, 417–421. [Google Scholar] [CrossRef]
- Srisomboon, S.; Kettratad, M.; Stray, A.; Pakawanit, P.; Rojviriya, C.; Patntirapong, S.; Panpisut, P. Effects of Silver Diamine Nitrate and Silver Diamine Fluoride on Dentin Remineralization and Cytotoxicity to Dental Pulp Cells: An In Vitro Study. J. Funct. Biomater. 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, J.; Qin, H.; Long, J.; Lin, X.; Xie, F. Remineralization of human dentin type I collagen fibrils induced by carboxylated polyamidoamine dendrimer/amorphous calcium phosphate nanocomposite: An in vitro study. J. Biomater. Sci. Polym. Ed. 2022, 33, 668–686. [Google Scholar] [CrossRef] [PubMed]
- Di Foggia, M.; Prati, C.; Gandolfi, M.G.; Taddei, P. An in vitro study on dentin demineralization and remineralization: Collagen rearrangements and influence on the enucleated phase. J. Inorg. Biochem. 2019, 193, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lynch, R.J.M.; Watson, T.F.; Banerjee, A. Chitosan-bioglass complexes promote subsurface remineralisation of incipient human carious enamel lesions. J. Dent. 2019, 84, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Boyes, V.; Festy, F.; Lynch, R.J.M.; Watson, T.F.; Banerjee, A. In-vitro subsurface remineralisation of artificial enamel white spot lesions pre-treated with chitosan. Dent. Mater. 2018, 34, 1154–1167. [Google Scholar] [CrossRef]
- Heukamp, J.; Korbmacher-Steiner, H.; Schmidt, S.; Neumann, C.M.; Bottenberg, P.; Jablonski-Momeni, A. Remineralisation capability of silver diamine fluoride in artificial enamel lesions on smooth surfaces using quantitative light-induced fluorescence measurements in-vitro. Sci. Rep. 2022, 12, 8498. [Google Scholar] [CrossRef]
- Alhothali, M.M.; Exterkate, R.A.M.; Lagerweij, M.D.; van Strijp, A.J.P.; Buijs, M.J.; van Loveren, C. The Effect of Various Fluoride Products on Dentine Lesions during pH-Cycling. Caries Res. 2022, 56, 64–72. [Google Scholar] [CrossRef]
- Amaechi, B.T.; AbdulAzees, P.A.; Okoye, L.O.; Meyer, F.; Enax, J. Comparison of hydroxyapatite and fluoride oral care gels for remineralization of initial caries: A pH-cycling study. BDJ Open 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Reise, M.; Kranz, S.; Heyder, M.; Jandt, K.D.; Sigusch, B.W. Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Compared to Fluoride Products in an In-Vitro Demineralization Model. Materials 2021, 14, 5974. [Google Scholar] [CrossRef] [PubMed]
- Aref, N.S.; Alsdrani, R.M. Surface topography and spectrophotometric assessment of white spot lesions restored with nano-hydroxyapatite-containing universal adhesive resin: An in-vitro study. BMC Oral Health 2023, 23, 911. [Google Scholar] [CrossRef] [PubMed]
- Aref, N.S.; Alrasheed, M.K. Casein phosphopeptide amorphous calcium phosphate and universal adhesive resin as a complementary approach for management of white spot lesions: An in-vitro study. Prog. Orthod. 2022, 23, 10. [Google Scholar] [CrossRef]
- Talwar, M.; Borzabadi-Farahani, A.; Lynch, E.; Borsboom, P.; Ruben, J. Remineralization of Demineralized Enamel and Dentine Using 3 Dentifrices-An InVitro Study. Dent. J. 2019, 7, 91. [Google Scholar] [CrossRef]
- Palmer, R.J., Jr. Composition and development of oral bacterial communities. Periodontol. 2000 2014, 64, 20–39. [Google Scholar] [CrossRef]
- Sissons, C.; Anderson, S.; Wong, L.; Coleman, M.; White, D. Microbiota of plaque microcosm biofilms: Effect of three times daily sucrose pulses in different simulated oral environments. Caries Res. 2007, 41, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Buzalaf, M.A.; Hannas, A.R.; Magalhães, A.C.; Rios, D.; Honório, H.M.; Delbem, A.C. pH-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: Strengths and limitations. J. Appl. Oral Sci. 2010, 18, 316–334. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Sousa, E.B.; Fernandes, N.L.; Meira, I.A.; Lavôr, J.R.; Chaves, A.M.; Sampaio, F.C. Effect of treatment time on performance of nano-encapsulated fluoride dentifrices for remineralization of initial carious lesions: An in vitro study. Acta Odontol. Latinoam. 2021, 34, 56–62. [Google Scholar] [CrossRef]
- Ghelejkhani, A.; Nadalizadeh, S.; Rajabi, M. Effect of casein-phosphopeptide amorphous calcium phosphate and fluoride with/without erbium, chromium-doped yttrium, scandium, gallium, and garnet laser irradiation on enamel microhardness of permanent teeth. Dent. Res. J. 2021, 18, 20. [Google Scholar] [CrossRef]
- Gonçalves, F.M.C.; Delbem, A.C.B.; Gomes, L.F.; Emerenciano, N.G.; Dos Passos Silva, M.; Cannon, M.L.; Danelon, M. Combined effect of casein phosphopeptide-amorphous calcium phosphate and sodium trimetaphosphate on the prevention of enamel demineralization and dental caries: An in vitro study. Clin. Oral Investig. 2021, 25, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Wierichs, R.J.; Rupp, K.; Meyer-Lueckel, H.; Apel, C.; Esteves-Oliveira, M. Effects of Dentifrices Differing in Fluoride Content on Remineralization Characteristics of Dentin in vitro. Caries Res. 2020, 54, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Premnath, P.; John, J.; Manchery, N.; Subbiah, G.K.; Nagappan, N.; Subramani, P. Effectiveness of Theobromine on Enamel Remineralization: A Comparative In-vitro Study. Cureus 2019, 11, e5686. [Google Scholar] [CrossRef]
- Alcorn, A.; Al Dehailan, L.; Cook, N.B.; Tang, Q.; Lippert, F. Longitudinal In Vitro Effects of Silver Diamine Fluoride on Early Enamel Caries Lesions. Oper. Dent. 2022, 47, 309–319. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Stausberg, S.; Lausch, J.; Meyer-Lueckel, H.; Esteves-Oliveira, M. Caries-Preventive Effect of NaF, NaF plus TCP, NaF plus CPP-ACP, and SDF Varnishes on Sound Dentin and Artificial Dentin Caries in vitro. Caries Res. 2018, 52, 199–211. [Google Scholar] [CrossRef]
- White, D.J. Reactivity of fluoride dentifrices with artificial caries. I. Effects on early lesions: F uptake, surface hardening and remineralization. Caries Res. 1987, 21, 126–140. [Google Scholar] [CrossRef]
- Walther, C.; Kreibohm, M.; Paris, S.; Meyer-Lueckel, H.; Tschoppe, P.; Wierichs, R.J. Effect of NaF, AmF, KF gels and NaF toothpaste combined with a saliva substitute on dentin lesions in vitro. Clin. Oral Investig. 2019, 23, 2489–2496. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Effect of strontium-doped bioactive glass-ceramic containing toothpaste on prevention of artificial dentine caries formation: An in vitro study. BMC Oral Health 2022, 22, 288. [Google Scholar] [CrossRef]
- Yin, I.X.; Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Li, Q.L.; Tang, J.; Lo, E.C.M.; Chu, C.H. Inhibition of dentine caries using fluoride solution with silver nanoparticles: An in vitro study. J. Dent. 2020, 103, 103512. [Google Scholar] [CrossRef]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.-L.; Mei, M.L.; Chu, C.H. Efficacy of the dual-action GA-KR12 peptide for remineralising initial enamel caries: An in vitro study. Clin. Oral Investig. 2022, 26, 2441–2451. [Google Scholar] [CrossRef]
- Klimek, J.; Hellwig, E.; Ahrens, G. Fluoride taken up by plaque, by the underlying enamel and by clean enamel from three fluoride compounds in vitro. Caries Res. 1982, 16, 156–161. [Google Scholar] [CrossRef]
- Akgun, O.M.; Haman Bayari, S.; Ide, S.; Guven Polat, G.; Yildirim, C.; Orujalipoor, I. Evaluation of the protective effect on enamel demineralization of CPP-ACP paste and ROCS by vibrational spectroscopy and SAXS: An in vitro study. Microsc. Res. Tech. 2021, 84, 2977–2987. [Google Scholar] [CrossRef]
- Siqueira, W.L.; Custodio, W.; McDonald, E.E. New Insights into the Composition and Functions of the Acquired Enamel Pellicle. J. Dent. Res. 2012, 91, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Ash, A.; Ridout, M.J.; Parker, R.; Mackie, A.R.; Burnett, G.R.; Wilde, P.J. Effect of calcium ions on in vitro pellicle formation from parotid and whole saliva. Colloids Surf. B Biointerfaces 2013, 102, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.; Gohil, U.; Parekh, V.; Joshi, S. Comparative Evaluation of the Remineralizing Potential of Commercially Available Agents on Artificially Demineralized Human Enamel: An In vitro Study. Contemp. Clin. Dent. 2019, 10, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyńska, M.; Voelkel, A. Stability of simulated body fluids such as blood plasma, artificial urine and artificial saliva. Microchem. J. 2017, 134, 197–201. [Google Scholar] [CrossRef]
- Rai, R.U.; Ranjan, R.; Kumar, M.; Mukri, U.; Mala, N.; Kumar, K. Remineralization of Artificial Dentin Lesion In vitro using Dental Nanomaterials. J. Pharm. Bioallied Sci. 2021, 13, S229–S232. [Google Scholar] [CrossRef]
- Lale, S.; Solak, H.; Hınçal, E.; Vahdettin, L. In Vitro Comparison of Fluoride, Magnesium, and Calcium Phosphate Materials on Prevention of White Spot Lesions around Orthodontic Brackets. Biomed. Res. Int. 2020, 2020, 1989817. [Google Scholar] [CrossRef]
- Maravic, T.; Mazzitelli, C.; Josic, U.; D’Alessandro, C.; Florenzano, F.; Generali, L.; Checchi, V.; Mazzoni, A.; Breschi, L. M3—Silver-diamine-fluoride (SDF) as Therapeutic Dentin Primer: 1-year In-vitro Evaluation. Dent. Mater. 2023, 39, e2. [Google Scholar] [CrossRef]

| Demineralisation Solution | pH | Exposure Time | Number of Articles (n) | Percentage (%) of Demineralisation Solution (n = 109) |
|---|---|---|---|---|
| Acetic acid buffer | 3.5–5.0 | - | 48 | 44.0% |
| 2–3 days | 8 | 7.3% | ||
| 4 days | 24 | 22.0% | ||
| 5–14 days | 7 | 6.4% | ||
| 2–10 weeks | 5 | 4.6% | ||
| Lactic acid buffer | 3.5–5.0 | - | 40 | 36.7% |
| 2 min–36 h | 3 | 2.8% | ||
| 2–4 days | 25 | 22.9% | ||
| 5–14 days | 9 | 8.3% | ||
| 2–3 weeks | 3 | 2.8% | ||
| pH cycling | - | 2–30 days | 12 | 11.0% |
| McInne’s solution | - | 5 min | 3 | 2.8% |
| EDTA solution | 8.0 | 30 min | 2 | 1.8% |
| HCl | <1 | 30 s | 2 | 1.8% |
| Coca-Cola | 2.52 | 20 s | 1 | 0.9% |
| Silverstone’s cariogenic solution | 4.3 | 4 weeks | 1 | 0.9% |
| Acid gel | pH | Exposure Time | Number of Articles (n) | Percentage (%) of Acid Gels (n = 29) |
| 37% phosphoric acid gel | <1 | 15 s | 16 | 55.2% |
| HEC gel | 4.95–5.1 | 10 days | 4 | 13.8% |
| 8 wt% MC gel | 4.6 | 10–21 days | 4 | 13.8% |
| 17% EDTA gel | 7.3 | 30 min–2 weeks | 3 | 10.3% |
| 6 wt% CMC gel | 5.0 | 3 weeks | 1 | 3.4% |
| Lactic acid gel | 5.0 | 24 h | 1 | 3.4% |
| Duration of pH Cycling | Number of Articles (n) | Percentage (%) of pH Cycling Studies (n = 88) |
|---|---|---|
| 3 days | 1 | 1.1% |
| 5 days | 7 | 8.0% |
| 6 days | 4 | 4.5% |
| 7 days | 24 | 27.3% |
| 8 days | 6 | 6.8% |
| 9 days | 2 | 2.3% |
| 10 days | 11 | 12.5% |
| 12 days | 5 | 5.7% |
| 14 days | 10 | 11.4% |
| 15 days | 1 | 1.1% |
| 19 days | 1 | 1.1% |
| 21 days | 4 | 4.5% |
| 28 days | 8 | 9.1% |
| 35 days | 1 | 1.1% |
| 1 or 3 months | 1 | 1.1% |
| Demineralisation | Remineralisation | Cycles per Day | Remineralisation Overnight | Number of Articles (n) | Percentage (%) of pH Cycling Studies (n = 88) |
|---|---|---|---|---|---|
| 3 h DS + 2 h RS + 3 h DS | 1 | 16 h | 9 | 10.2% | |
| 4 h DS + 6 h TS + 14 h RS | 1 | - | 1 | 1.1% | |
| 14 h DS + 2 h TS + 8 h RS | 1 | - | 1 | 1.1% | |
| 16 h | 8 h | 1 | - | 1 | 1.1% |
| 8 h | 16 h | 1 | - | 11 | 12.5% |
| 6 h | 18 h | 1 | - | 15 | 17.0% |
| 6 h | 17 h | 1 | - | 2 | 2.3% |
| 6 h | 16 h | 1 | - | 1 | 1.1% |
| 4 h | 20 h | 1 | - | 7 | 8.0% |
| 3 h | 21 h | 1 | - | 9 | 10.2% |
| 3 h | 17 h | 1 | - | 1 | 1.1% |
| 2 h | 22 h | 1 | - | 13 | 14.8% |
| 3 h | 3 h | 2 | 12 h | 1 | 1.1% |
| 1 h | 11 h | 2 | - | 1 | 1.1% |
| 10 min | 5 min | 2 | - | 1 | 1.1% |
| 1 h | 0.5 h | 4 | 18 h | 1 | 1.1% |
| 2 h | 1 h | 6 | 6 h | 2 | 2.3% |
| 1 h | 2 h | 6 | 6 h | 2 | 2.3% |
| 1 h | 2 h | 6 | 6 h | 1 | 1.1% |
| 0.5 h | 2.5 h | 6 | 6 h | 2 | 2.3% |
| Simple Chemical Models | pH | Exposure Time | Number of Articles (n) | Percentage (%) of Simple Chemical Models (n = 88) |
|---|---|---|---|---|
| Artificial saliva | 6.8–7.2 | 5–30 days | 62 | 88.6% |
| Remineralisation solution | 6.8–7.2 | 7–28 days | 6 | 8.6% |
| Simulated body fluid | 7.4 | 2 weeks, 38 days | 2 | 2.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Ekambaram, M.; Li, K.C.; Zhang, Y.; Cooper, P.R.; Mei, M.L. In Vitro Models Used in Cariology Mineralisation Research—A Review of the Literature. Dent. J. 2024, 12, 323. https://doi.org/10.3390/dj12100323
Fu Y, Ekambaram M, Li KC, Zhang Y, Cooper PR, Mei ML. In Vitro Models Used in Cariology Mineralisation Research—A Review of the Literature. Dentistry Journal. 2024; 12(10):323. https://doi.org/10.3390/dj12100323
Chicago/Turabian StyleFu, Yipeng, Manikandan Ekambaram, Kai Chun Li, Ya Zhang, Paul R. Cooper, and May Lei Mei. 2024. "In Vitro Models Used in Cariology Mineralisation Research—A Review of the Literature" Dentistry Journal 12, no. 10: 323. https://doi.org/10.3390/dj12100323
APA StyleFu, Y., Ekambaram, M., Li, K. C., Zhang, Y., Cooper, P. R., & Mei, M. L. (2024). In Vitro Models Used in Cariology Mineralisation Research—A Review of the Literature. Dentistry Journal, 12(10), 323. https://doi.org/10.3390/dj12100323







