The Role of Dental Practitioners in the Management of Oncology Patients: The Head and Neck Radiation Oncology Patient and the Medical Oncology Patient
Abstract
1. Introduction
1.1. Oral Mucositis
Prevention and Management of Oral Mucositis
1.2. Trismus
Prevention and Management of Trismus
1.3. Xerostomia/Hyposalivation
Prevention and Management of RT-Induced Xerostomia
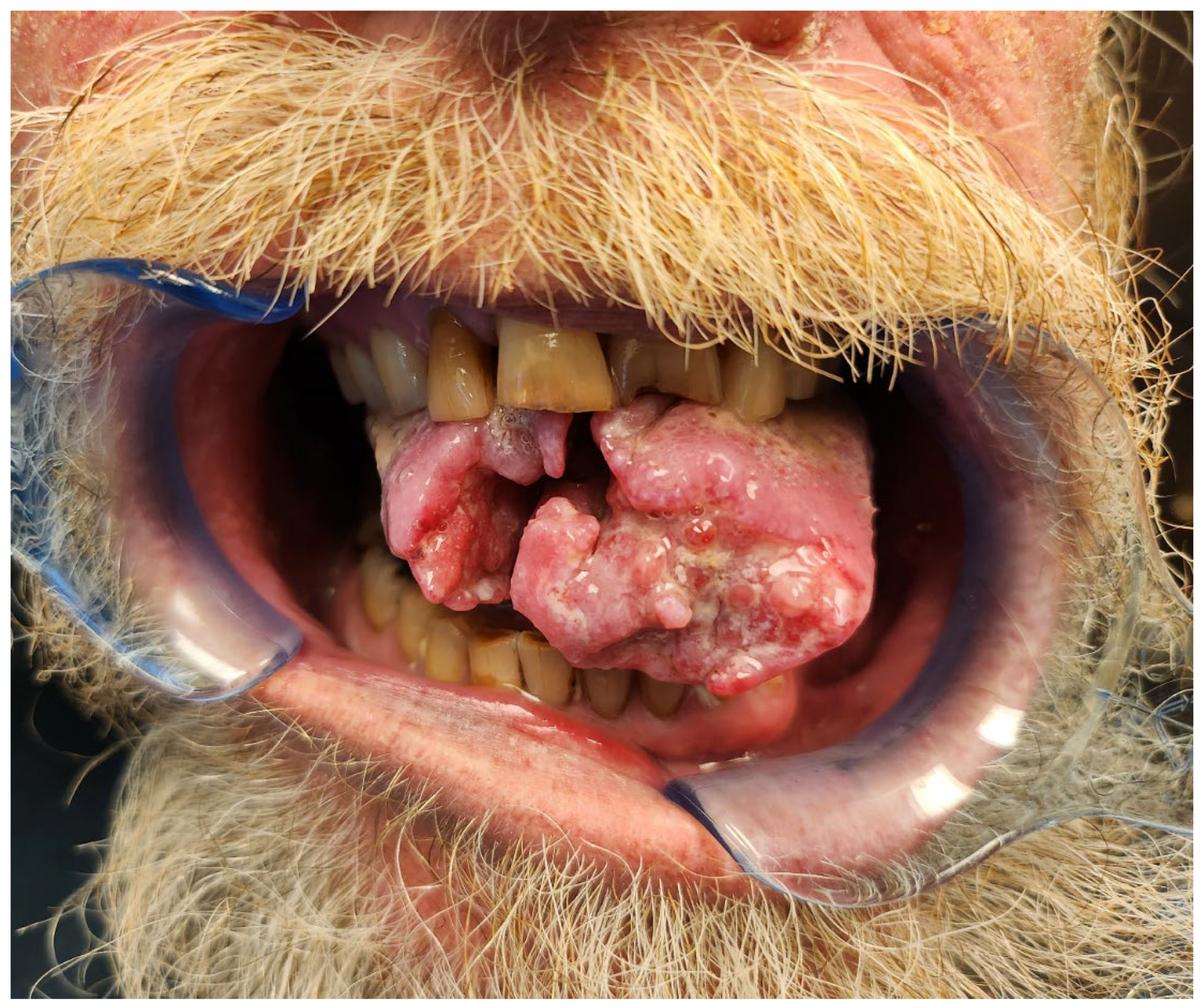
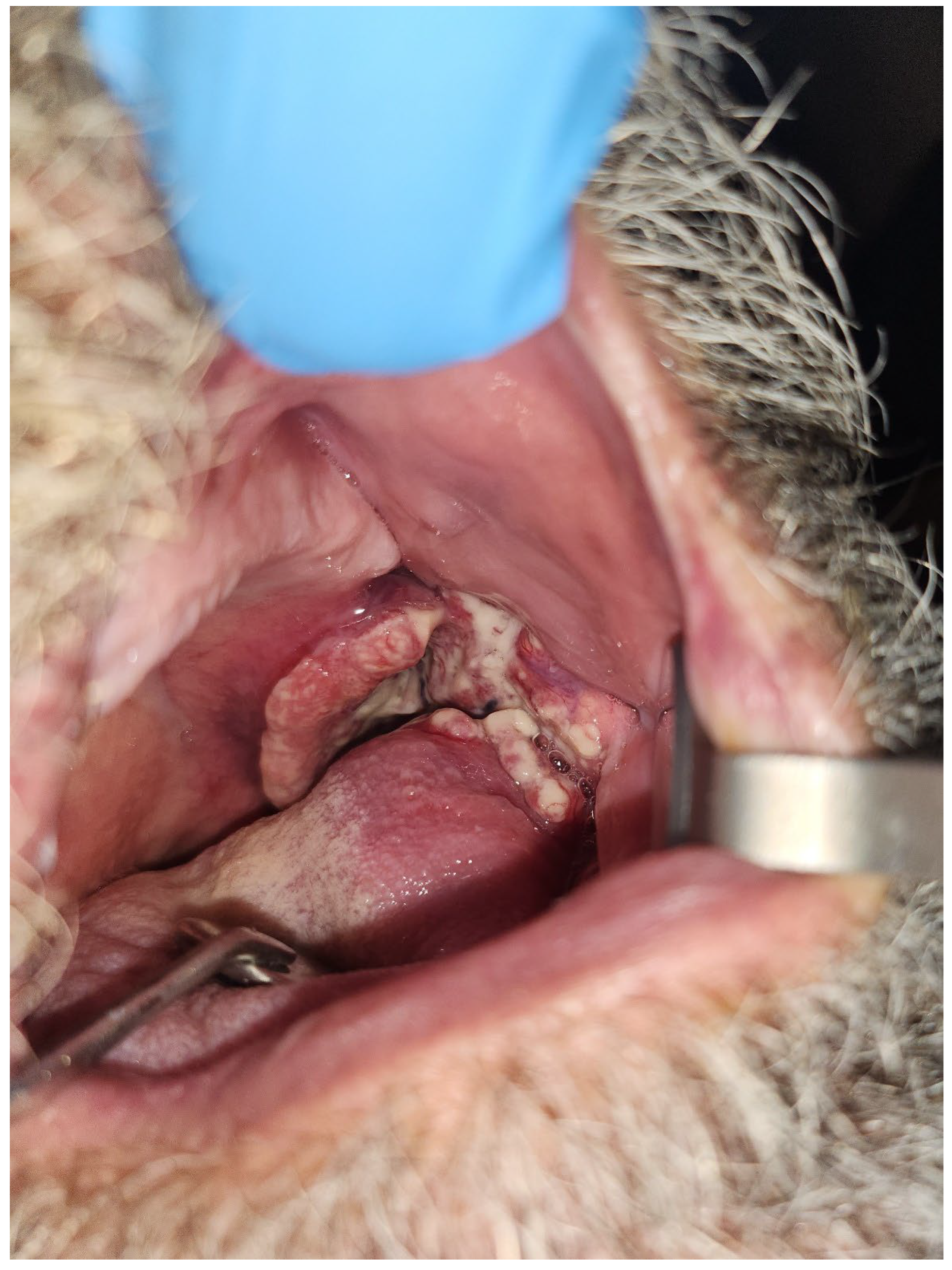
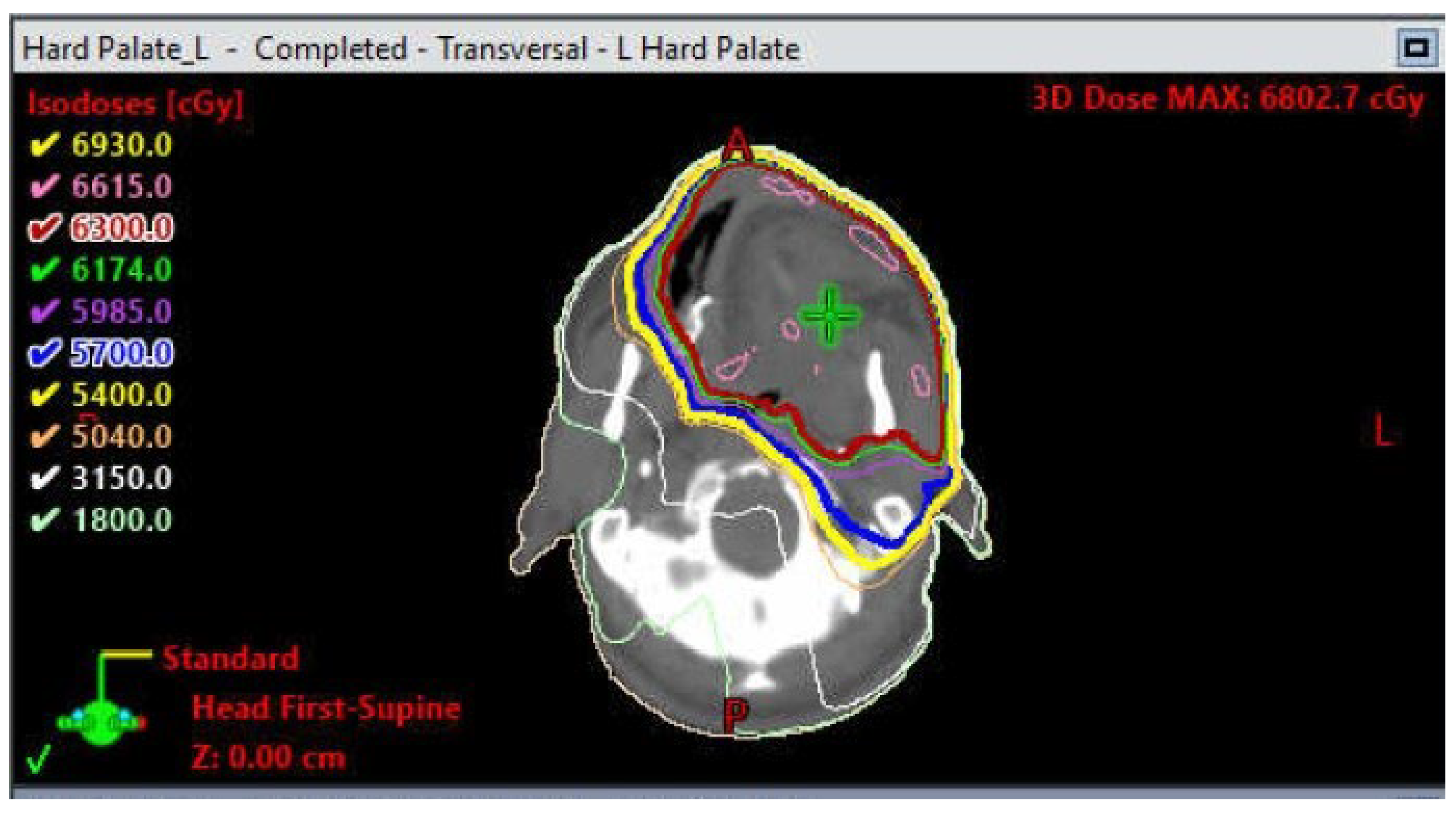
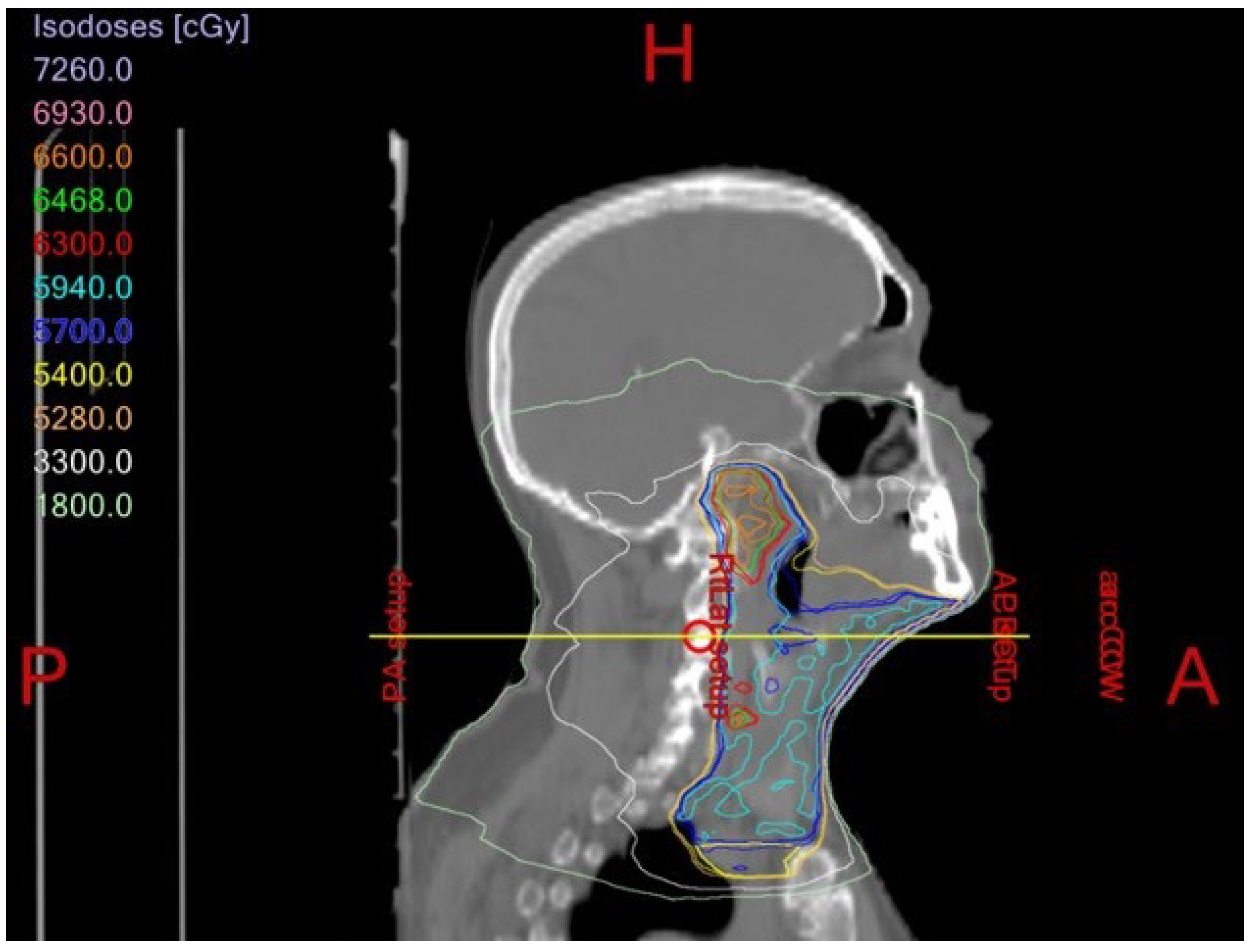
1.4. Osteoradionecrosis
Prevention and Management of Osteoradionecrosis
1.5. Abnormal Development of Orofacial Structures
Prevention and Management of Abnormal Development of Orofacial Structures
2. Oncology Patients on Antiresorptive/Antiangiogenic Medications
2.1. Prevention and Management of MRONJ
2.2. Hematopoietic Stem Cell Transplant (HSCT)
2.3. Immune Checkpoint Inhibitors (Immunotherapy)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Cahlon, O.; Scher, E.; Zhou, Y.; Berry, S.L.; Rybkin, A.; Sine, K.M.; Tang, S.; Sherman, E.J.; Wong, R.; et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother. Oncol. 2016, 118, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lohia, S.; Rajapurkar, M.; Nguyen, S.A.; Sharma, A.K.; Gillespie, M.B.; Day, T.A. A comparison of outcomes using intensity-modulated radiation therapy and 3-dimensional conformal radiation therapy in treatment of oropharyngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B., 2nd; Ly, D.; Dan, T.D.; Ondos, J.; Ning, H.; Belard, A.; O’Connell, J.; Miller, R.W.; Simone, N.L. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother. Oncol. 2011, 101, 376–382. [Google Scholar] [CrossRef]
- Owosho, A.A.; Yom, S.K.; Han, Z.; Sine, K.; Lee, N.Y.; Huryn, J.M.; Estilo, C.L. Comparison of mean radiation dose and dosimetric distribution to tooth-bearing regions of the mandible associated with proton beam radiation therapy and intensity-modulated radiation therapy for ipsilateral head and neck tumor. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 566–571. [Google Scholar] [CrossRef]
- Franco, P.; Martini, S.; Di Muzio, J.; Cavallin, C.; Arcadipane, F.; Rampino, M.; Ostellino, O.; Pecorari, G.; Garzino Demo, P.; Fasolis, M.; et al. Prospective assessment of oral mucositis and its impact on quality of life and patient-reported outcomes during radiotherapy for head and neck cancer. Med. Oncol. 2017, 34, 81. [Google Scholar] [CrossRef]
- Owosho, A.A.; Pedreira Ramalho, L.M.; Rosenberg, H.I.; Yom, S.K.; Drill, E.; Riedel, E.; Tsai, C.J.; Lee, N.Y.; Huryn, J.M.; Estilo, C.L. Objective assessment of trismus in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT). J. Cranio-Maxillofac. Surg. 2016, 44, 1408–1413. [Google Scholar] [CrossRef]
- Owosho, A.A.; Thor, M.; Oh, J.H.; Riaz, N.; Tsai, C.J.; Rosenberg, H.; Varthis, S.; Yom, S.H.; Huryn, J.M.; Lee, N.Y.; et al. The role of parotid gland irradiation in the development of severe hyposalivation (xerostomia) after intensity-modulated radiation therapy for head and neck cancer: Temporal patterns, risk factors, and testing the QUANTEC guidelines. J. Cranio-Maxillofac. Surg. 2017, 45, 595–600. [Google Scholar] [CrossRef]
- Singh, A.; Kitpanit, S.; Neal, B.; Yorke, E.; White, C.; Yom, S.K.; Randazzo, J.D.; Wong, R.J.; Huryn, J.M.; Tsai, C.J.; et al. Osteoradionecrosis of the Jaw Following Proton Radiation Therapy for Patients with Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2022, 149, 151. [Google Scholar] [CrossRef]
- Owosho, A.A.; Tsai, C.J.; Lee, R.S.; Freymiller, H.; Kadempour, A.; Varthis, S.; Sax, A.Z.; Rosen, E.B.; Yom, S.K.; Randazzo, J.; et al. The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT): The Memorial Sloan Kettering Cancer Center experience. Oral Oncol. 2017, 64, 44–51. [Google Scholar] [CrossRef]
- Owosho, A.A.; Brady, P.; Wolden, S.L.; Wexler, L.H.; Antonescu, C.R.; Huryn, J.M.; Estilo, C.L. Long-term effect of chemotherapy-intensity-modulated radiation therapy (chemo-IMRT) on dentofacial development in head and neck rhabdomyosarcoma patients. Pediatr. Hematol. Oncol. 2016, 33, 383–392. [Google Scholar] [CrossRef]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B.; et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Antonadou, D.; Pepelassi, M.; Synodinou, M.; Puglisi, M.; Throuvalas, N. Prophylactic use of amifostine to prevent radiochemotherapy-induced mucositis and xerostomia in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 739–747. [Google Scholar] [CrossRef]
- Rades, D.; Fehlauer, F.; Bajrovic, A.; Mahlmann, B.; Richter, E.; Alberti, W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother. Oncol. 2004, 70, 261–264. [Google Scholar] [CrossRef]
- Oberoi, S.; Zamperlini-Netto, G.; Beyene, J.; Treister, N.S.; Sung, L. Effect of prophylactic low level laser therapy on oral mucositis: A systematic review and meta-analysis. PLoS ONE 2014, 9, e107418. [Google Scholar] [CrossRef]
- Peng, J.; Shi, Y.; Wang, J.; Wang, F.; Dan, H.; Xu, H.; Zeng, X. Low-level laser therapy in the prevention and treatment of oral mucositis: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 387–397.e389. [Google Scholar] [CrossRef]
- Amiri Khosroshahi, R.; Talebi, S.; Travica, N.; Mohammadi, H. Cryotherapy for oral mucositis in cancer: Review of systematic reviews and meta-analysis. BMJ Support. Palliat. Care 2022. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Tu, Y.K.; Ding, Y.W.; Lin, J.J. Effectiveness of low level laser therapy versus cryotherapy in cancer patients with oral mucositis: Systematic review and network meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 160, 103276. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Bensadoun, R.J.; Riesenbeck, D.; Lockhart, P.B.; Elting, L.S.; Spijkervet, F.K.; Brennan, M.T.; Trismus Section, Oral Care Study Group. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support. Care Cancer 2010, 18, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, P.U.; Kalk, W.W.; Roodenburg, J.L. Trismus in head and neck oncology: A systematic review. Oral Oncol. 2004, 40, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, P.U.; Huisman, P.M.; Roodenburg, J.L. Criteria for trismus in head and neck oncology. Int. J. Oral Maxillofac. Surg. 2006, 35, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Louise Kent, M.; Brennan, M.T.; Noll, J.L.; Fox, P.C.; Burri, S.H.; Hunter, J.C.; Lockhart, P.B. Radiation-induced trismus in head and neck cancer patients. Support. Care Cancer 2008, 16, 305–309. [Google Scholar] [CrossRef]
- Pauli, N.; Johnson, J.; Finizia, C.; Andrell, P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol. 2013, 52, 1137–1145. [Google Scholar] [CrossRef]
- Lee, L.Y.; Chen, S.C.; Chen, W.C.; Huang, B.S.; Lin, C.Y. Postradiation trismus and its impact on quality of life in patients with head and neck cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 187–195. [Google Scholar] [CrossRef]
- Gomez, D.R.; Zhung, J.E.; Gomez, J.; Chan, K.; Wu, A.J.; Wolden, S.L.; Pfister, D.G.; Shaha, A.; Shah, J.P.; Kraus, D.H.; et al. Intensity-modulated radiotherapy in postoperative treatment of oral cavity cancers. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1096–1103. [Google Scholar] [CrossRef]
- Chao, K.S.; Ozyigit, G.; Blanco, A.I.; Thorstad, W.L.; Deasy, J.O.; Haughey, B.H.; Spector, G.J.; Sessions, D.G. Intensity-modulated radiation therapy for oropharyngeal carcinoma: Impact of tumor volume. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 43–50. [Google Scholar] [CrossRef]
- Ingle, C.J.; Yip, K.; Caskie, V.; Dyson, C.; Ford, A.; Scrase, C.D. Intensity modulated radiotherapy (IMRT) in the management of locally advanced oropharyngeal squamous cell carcinomata (SCC): Disease control and functional outcome using the therapy outcome measure (TOM) score-report from a single U.K. institution. Head Neck Oncol. 2010, 2, 28. [Google Scholar] [CrossRef]
- Hsieh, L.C.; Chen, J.W.; Wang, L.Y.; Tsang, Y.M.; Shueng, P.W.; Liao, L.J.; Lo, W.C.; Lin, Y.C.; Tseng, C.F.; Kuo, Y.S.; et al. Predicting the severity and prognosis of trismus after intensity-modulated radiation therapy for oral cancer patients by magnetic resonance imaging. PLoS ONE 2014, 9, e92561. [Google Scholar] [CrossRef]
- Rao, S.D.; Saleh, Z.H.; Setton, J.; Tam, M.; McBride, S.M.; Riaz, N.; Deasy, J.O.; Lee, N.Y. Dose-volume factors correlating with trismus following chemoradiation for head and neck cancer. Acta Oncol. 2016, 55, 99–104. [Google Scholar] [CrossRef]
- Kamstra, J.I.; Dijkstra, P.U.; van Leeuwen, M.; Roodenburg, J.L.; Langendijk, J.A. Mouth opening in patients irradiated for head and neck cancer: A prospective repeated measures study. Oral Oncol. 2015, 51, 548–555. [Google Scholar] [CrossRef]
- Lindblom, U.; Garskog, O.; Kjellen, E.; Laurell, G.; Levring Jaghagen, E.; Wahlberg, P.; Zackrisson, B.; Nilsson, P. Radiation-induced trismus in the ARTSCAN head and neck trial. Acta Oncol. 2014, 53, 620–627. [Google Scholar] [CrossRef]
- Teguh, D.N.; Levendag, P.C.; Voet, P.; van der Est, H.; Noever, I.; de Kruijf, W.; van Rooij, P.; Schmitz, P.I.; Heijmen, B.J. Trismus in patients with oropharyngeal cancer: Relationship with dose in structures of mastication apparatus. Head Neck 2008, 30, 622–630. [Google Scholar] [CrossRef]
- Kamstra, J.I.; van Leeuwen, M.; Roodenburg, J.L.N.; Dijkstra, P.U. Exercise therapy for trismus secondary to head and neck cancer: A systematic review. Head Neck 2017, 39, 2352–2362. [Google Scholar] [CrossRef]
- Dijkstra, P.U.; Sterken, M.W.; Pater, R.; Spijkervet, F.K.; Roodenburg, J.L. Exercise therapy for trismus in head and neck cancer. Oral Oncol. 2007, 43, 389–394. [Google Scholar] [CrossRef]
- Dijkstra, P.U. Dynasplint for the management of trismus after treatment of upper aerodigestive tract cancer: A retrospective study. Ear. Nose Throat J. 2012, 91, E35. [Google Scholar]
- Kamstra, J.I.; Roodenburg, J.L.; Beurskens, C.H.; Reintsema, H.; Dijkstra, P.U. TheraBite exercises to treat trismus secondary to head and neck cancer. Support. Care Cancer 2013, 21, 951–957. [Google Scholar] [CrossRef]
- Bjordal, K.; Kaasa, S.; Mastekaasa, A. Quality of life in patients treated for head and neck cancer: A follow-up study 7 to 11 years after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 847–856. [Google Scholar] [CrossRef]
- Wijers, O.B.; Levendag, P.C.; Braaksma, M.M.; Boonzaaijer, M.; Visch, L.L.; Schmitz, P.I. Patients with head and neck cancer cured by radiation therapy: A survey of the dry mouth syndrome in long-term survivors. Head Neck 2002, 24, 737–747. [Google Scholar] [CrossRef]
- Coppes, R.P.; Zeilstra, L.J.; Kampinga, H.H.; Konings, A.W. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br. J. Cancer 2001, 85, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.M.; Brunsting, J.F.; Wierenga, P.K.; Faber, H.; Stokman, M.A.; Kok, T.; Visser, W.H.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE 2008, 3, e2063. [Google Scholar] [CrossRef] [PubMed]
- Chao, K.S.; Deasy, J.O.; Markman, J.; Haynie, J.; Perez, C.A.; Purdy, J.A.; Low, D.A. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: Initial results. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M.D.; Dische, S. The early changes in salivary gland function during and after radiotherapy given for head and neck cancer. Radiother. Oncol. 1994, 30, 26–32. [Google Scholar] [CrossRef]
- Blanco, A.I.; Chao, K.S.; El Naqa, I.; Franklin, G.E.; Zakarian, K.; Vicic, M.; Deasy, J.O. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1055–1069. [Google Scholar] [CrossRef]
- Beetz, I.; Steenbakkers, R.J.; Chouvalova, O.; Leemans, C.R.; Doornaert, P.; van der Laan, B.F.; Christianen, M.E.; Vissink, A.; Bijl, H.P.; van Luijk, P.; et al. The QUANTEC criteria for parotid gland dose and their efficacy to prevent moderate to severe patient-rated xerostomia. Acta Oncol. 2014, 53, 597–604. [Google Scholar] [CrossRef]
- Moiseenko, V.; Wu, J.; Hovan, A.; Saleh, Z.; Apte, A.; Deasy, J.O.; Harrow, S.; Rabuka, C.; Muggli, A.; Thompson, A. Treatment planning constraints to avoid xerostomia in head-and-neck radiotherapy: An independent test of QUANTEC criteria using a prospectively collected dataset. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1108–1114. [Google Scholar] [CrossRef]
- Cheng, C.Q.; Xu, H.; Liu, L.; Wang, R.N.; Liu, Y.T.; Li, J.; Zhou, X.K. Efficacy and safety of pilocarpine for radiation-induced xerostomia in patients with head and neck cancer: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2016, 147, 236–243. [Google Scholar] [CrossRef]
- Mercadante, V.; Jensen, S.B.; Smith, D.K.; Bohlke, K.; Bauman, J.; Brennan, M.T.; Coppes, R.P.; Jessen, N.; Malhotra, N.K.; Murphy, B.; et al. Salivary Gland Hypofunction and/or Xerostomia Induced by Nonsurgical Cancer Therapies: ISOO/MASCC/ASCO Guideline. J. Clin. Oncol. 2021, 39, 2825–2843. [Google Scholar] [CrossRef]
- Marx, R.E. Osteoradionecrosis: A new concept of its pathophysiology. J. Oral Maxillofac. Surg. 1983, 41, 283–288. [Google Scholar] [CrossRef]
- Epstein, J.B.; Wong, F.L.; Stevenson-Moore, P. Osteoradionecrosis: Clinical experience and a proposal for classification. J. Oral Maxillofac. Surg. 1987, 45, 104–110. [Google Scholar] [CrossRef]
- van Merkesteyn, J.P.; Balm, A.J.; Bakker, D.J.; Borgmeyer-Hoelen, A.M. Hyperbaric oxygen treatment of osteoradionecrosis of the mandible with repeated pathologic fracture. Report of a case. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 461–464. [Google Scholar] [CrossRef]
- Store, G.; Boysen, M. Mandibular osteoradionecrosis: Clinical behaviour and diagnostic aspects. Clin. Otolaryngol. Allied Sci. 2000, 25, 378–384. [Google Scholar] [CrossRef]
- Lee, I.J.; Koom, W.S.; Lee, C.G.; Kim, Y.B.; Yoo, S.W.; Keum, K.C.; Kim, G.E.; Choi, E.C.; Cha, I.H. Risk factors and dose-effect relationship for mandibular osteoradionecrosis in oral and oropharyngeal cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1084–1091. [Google Scholar] [CrossRef]
- Mendenhall, W.M. Mandibular osteoradionecrosis. J. Clin. Oncol. 2004, 22, 4867–4868. [Google Scholar] [CrossRef]
- Oh, H.K.; Chambers, M.S.; Martin, J.W.; Lim, H.J.; Park, H.J. Osteoradionecrosis of the mandible: Treatment outcomes and factors influencing the progress of osteoradionecrosis. J. Oral Maxillofac. Surg. 2009, 67, 1378–1386. [Google Scholar] [CrossRef]
- Wahl, M.J. Osteoradionecrosis prevention myths. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 661–669. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Zarra, T.; Troltzsch, M.; Mahaini, S.; Ehrenfeld, M.; Otto, S. Osteoradionecrosis of the mandible: A ten year single-center retrospective study. J. Cranio-Maxillofac. Surg. 2015, 43, 837–846. [Google Scholar] [CrossRef]
- Tsai, C.J.; Hofstede, T.M.; Sturgis, E.M.; Garden, A.S.; Lindberg, M.E.; Wei, Q.; Tucker, S.L.; Dong, L. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 415–420. [Google Scholar] [CrossRef]
- Studer, G.; Bredell, M.; Studer, S.; Huber, G.; Glanzmann, C. Risk profile for osteoradionecrosis of the mandible in the IMRT era. Strahlenther. Onkol. 2016, 192, 32–39. [Google Scholar] [CrossRef]
- Lukens, J.N.; Lin, A.; Hahn, S.M. Proton therapy for head and neck cancer. Curr. Opin. Oncol. 2015, 27, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Studer, G.; Gratz, K.W.; Glanzmann, C. Osteoradionecrosis of the mandibula in patients treated with different fractionations. Strahlenther. Onkol. 2004, 180, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.; Butterworth, C.; Tesfaye, B.; Bickerstaff, M.; Dodd, S.; Smerdon, G.; Chauhan, S.; Brennan, P.; Webster, K.; McCaul, J.; et al. HOPON (Hyperbaric Oxygen for the Prevention of Osteoradionecrosis): A randomised controlled trial of hyperbaric oxygen to prevent osteoradionecrosis of the irradiated mandible: Study protocol for a randomised controlled trial. Trials 2018, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Kolokythas, A.; Rasmussen, J.T.; Reardon, J.; Feng, C. Management of osteoradionecrosis of the jaws with pentoxifylline-tocopherol: A systematic review of the literature and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 173–180. [Google Scholar] [CrossRef]
- Ribeiro, G.H.; Minamisako, M.C.; Rath, I.; Santos, A.M.B.; Simoes, A.; Pereira, K.C.R.; Grando, L.J. Osteoradionecrosis of the jaws: Case series treated with adjuvant low-level laser therapy and antimicrobial photodynamic therapy. J. Appl. Oral Sci. 2018, 26, e20170172. [Google Scholar] [CrossRef]
- Cha, Y.H.; Hong, N.; Rhee, Y.; Cha, I.H. Teriparatide therapy for severe, refractory osteoradionecrosis of the jaw. Osteoporos. Int. 2018, 29, 987–992. [Google Scholar] [CrossRef]
- Hicks, J.; Flaitz, C. Rhabdomyosarcoma of the head and neck in children. Oral Oncol. 2002, 38, 450–459. [Google Scholar] [CrossRef]
- Maguire, A.; Craft, A.W.; Evans, R.G.; Amineddine, H.; Kernahan, J.; Macleod, R.I.; Murray, J.J.; Welbury, R.R. The long-term effects of treatment on the dental condition of children surviving malignant disease. Cancer 1987, 60, 2570–2575. [Google Scholar] [CrossRef]
- Raney, R.B.; Asmar, L.; Vassilopoulou-Sellin, R.; Klein, M.J.; Donaldson, S.S.; Green, J.; Heyn, R.; Wharam, M.; Glicksman, A.S.; Gehan, E.A.; et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: A descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and-III. IRS Group of the Children’s Cancer Group and the Pediatric Oncology Group. Med. Pediatr. Oncol. 1999, 33, 362–371. [Google Scholar]
- Kaste, S.C.; Hopkins, K.P.; Bowman, L.C. Dental abnormalities in long-term survivors of head and neck rhabdomyosarcoma. Med. Pediatr. Oncol. 1995, 25, 96–101. [Google Scholar] [CrossRef]
- Kaste, S.C.; Goodman, P.; Leisenring, W.; Stovall, M.; Hayashi, R.J.; Yeazel, M.; Beiraghi, S.; Hudson, M.M.; Sklar, C.A.; Robison, L.L.; et al. Impact of radiation and chemotherapy on risk of dental abnormalities: A report from the Childhood Cancer Survivor Study. Cancer 2009, 115, 5817–5827. [Google Scholar] [CrossRef]
- Kaste, S.C.; Hopkins, K.P.; Jenkins, J.J., 3rd. Abnormal odontogenesis in children treated with radiation and chemotherapy: Imaging findings. AJR Am. J. Roentgenol. 1994, 162, 1407–1411. [Google Scholar] [CrossRef]
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Rachner, T.D.; Coleman, R.E.; Jakob, F. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2014, 2, 500–512. [Google Scholar] [CrossRef]
- Fleissig, Y.; Regev, E.; Lehman, H. Sunitinib related osteonecrosis of jaw: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, e1–e3. [Google Scholar] [CrossRef]
- Estilo, C.L.; Fornier, M.; Farooki, A.; Carlson, D.; Bohle, G., 3rd; Huryn, J.M. Osteonecrosis of the jaw related to bevacizumab. J. Clin. Oncol. 2008, 26, 4037–4038. [Google Scholar] [CrossRef]
- Kim, D.W.; Jung, Y.S.; Park, H.S.; Jung, H.D. Osteonecrosis of the jaw related to everolimus: A case report. Br. J. Oral Maxillofac. Surg. 2013, 51, e302–e304. [Google Scholar] [CrossRef]
- Owosho, A.A.; Scordo, M.; Yom, S.K.; Randazzo, J.; Chapman, P.B.; Huryn, J.M.; Estilo, C.L. Osteonecrosis of the jaw a new complication related to Ipilimumab. Oral Oncol. 2015, 51, e100–e101. [Google Scholar] [CrossRef]
- Mawardi, H.; Enzinger, P.; McCleary, N.; Manon, R.; Villa, A.; Treister, N.; Woo, S.B. Osteonecrosis of the jaw associated with ziv-aflibercept. J. Gastrointest. Oncol. 2016, 7, E81–E87. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Kouri, M.; Papadopoulou, E.; Vardas, E.; Galiti, D.; Epstein, J.B.; Elad, S.; Campisi, G.; Tsoukalas, N.; Bektas-Kayhan, K.; et al. Osteonecrosis of the jaw related to non-antiresorptive medications: A systematic review. Support. Care Cancer 2019, 27, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Liang, S.T.Y.; Sax, A.Z.; Wu, K.; Yom, S.K.; Huryn, J.M.; Estilo, C.L. Medication-related osteonecrosis of the jaw: An update on the memorial sloan kettering cancer center experience and the role of premedication dental evaluation in prevention. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Hallmer, F.; Andersson, G.; Gotrick, B.; Warfvinge, G.; Anderud, J.; Bjornland, T. Prevalence, initiating factor, and treatment outcome of medication-related osteonecrosis of the jaw-a 4-year prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 477–485. [Google Scholar] [CrossRef]
- Saad, F.; Brown, J.E.; Van Poznak, C.; Ibrahim, T.; Stemmer, S.M.; Stopeck, A.T.; Diel, I.J.; Takahashi, S.; Shore, N.; Henry, D.H.; et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 2012, 23, 1341–1347. [Google Scholar] [CrossRef]
- Kyrgidis, A.; Vahtsevanos, K.; Koloutsos, G.; Andreadis, C.; Boukovinas, I.; Teleioudis, Z.; Patrikidou, A.; Triaridis, S. Bisphosphonate-related osteonecrosis of the jaws: A case-control study of risk factors in breast cancer patients. J. Clin. Oncol. 2008, 26, 4634–4638. [Google Scholar] [CrossRef]
- Mozzati, M.; Arata, V.; Gallesio, G. Tooth extraction in patients on zoledronic acid therapy. Oral Oncol. 2012, 48, 817–821. [Google Scholar] [CrossRef]
- Yamazaki, T.; Yamori, M.; Ishizaki, T.; Asai, K.; Goto, K.; Takahashi, K.; Nakayama, T.; Bessho, K. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: A cohort study. Int. J. Oral Maxillofac. Surg. 2012, 41, 1397–1403. [Google Scholar] [CrossRef]
- Scoletta, M.; Arata, V.; Arduino, P.G.; Lerda, E.; Chiecchio, A.; Gallesio, G.; Scully, C.; Mozzati, M. Tooth extractions in intravenous bisphosphonate-treated patients: A refined protocol. J. Oral Maxillofac. Surg. 2013, 71, 994–999. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Knauer, M.; Moik, M.; Jakesz, R.; Seifert, M.; Taucher, S.; Bjelic-Radisic, V.; et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: Final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann. Oncol. 2015, 26, 313–320. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef]
- Ng, T.L.; Tu, M.M.; Ibrahim, M.F.K.; Basulaiman, B.; McGee, S.F.; Srikanthan, A.; Fernandes, R.; Vandermeer, L.; Stober, C.; Sienkiewicz, M.; et al. Long-term impact of bone-modifying agents for the treatment of bone metastases: A systematic review. Support. Care Cancer 2021, 29, 925–943. [Google Scholar] [CrossRef]
- Bramati, A.; Girelli, S.; Farina, G.; Dazzani, M.C.; Torri, V.; Moretti, A.; Piva, S.; Dimaiuta, M.; La Verde, N. Prospective, mono-institutional study of the impact of a systematic prevention program on incidence and outcome of osteonecrosis of the jaw in patients treated with bisphosphonates for bone metastases. J. Bone Miner. Metab. 2015, 33, 119–124. [Google Scholar] [CrossRef]
- Bonacina, R.; Mariani, U.; Villa, F.; Villa, A. Preventive strategies and clinical implications for bisphosphonate-related osteonecrosis of the jaw: A review of 282 patients. J. Can. Dent. Assoc. 2011, 77, b147. [Google Scholar]
- Dimopoulos, M.A.; Kastritis, E.; Bamia, C.; Melakopoulos, I.; Gika, D.; Roussou, M.; Migkou, M.; Eleftherakis-Papaiakovou, E.; Christoulas, D.; Terpos, E.; et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann. Oncol. 2009, 20, 117–120. [Google Scholar] [CrossRef]
- Ripamonti, C.I.; Lucchesi, M.; Giusti, R. Prevention and management of osteonecrosis of the jaw secondary to bone-targeted therapy in patients with kidney cancer. Curr. Opin. Support. Palliat. Care 2016, 10, 273–280. [Google Scholar] [CrossRef]
- Epstein, M.S.; Wicknick, F.W.; Epstein, J.B.; Berenson, J.R.; Gorsky, M. Management of bisphosphonate-associated osteonecrosis: Pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 593–596. [Google Scholar] [CrossRef]
- Owosho, A.A.; Estilo, C.L.; Huryn, J.M.; Yom, S.K. Pentoxifylline and tocopherol in the management of cancer patients with medication-related osteonecrosis of the jaw: An observational retrospective study of initial case series. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 455–459. [Google Scholar] [CrossRef]
- Vescovi, P.; Meleti, M.; Merigo, E.; Manfredi, M.; Fornaini, C.; Guidotti, R.; Nammour, S. Case series of 589 tooth extractions in patients under bisphosphonates therapy. Proposal of a clinical protocol supported by Nd:YAG low-level laser therapy. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e680–e685. [Google Scholar] [CrossRef]
- Vescovi, P.; Giovannacci, I.; Merigo, E.; Meleti, M.; Manfredi, M.; Fornaini, C.; Nammour, S. Tooth extractions in high-risk patients under bisphosphonate therapy and previously affected with osteonecrosis of the jaws: Surgical protocol supported by low-level laser therapy. J. Craniofac. Surg. 2015, 26, 696–699. [Google Scholar] [CrossRef]
- Sim, I.W.; Borromeo, G.L.; Tsao, C.; Hardiman, R.; Hofman, M.S.; Papatziamos Hjelle, C.; Siddique, M.; Cook, G.J.R.; Seymour, J.F.; Ebeling, P.R. Teriparatide Promotes Bone Healing in Medication-Related Osteonecrosis of the Jaw: A Placebo-Controlled, Randomized Trial. J. Clin. Oncol. 2020, 38, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Yarom, N.; Shapiro, C.L.; Peterson, D.E.; Van Poznak, C.H.; Bohlke, K.; Ruggiero, S.L.; Migliorati, C.A.; Khan, A.; Morrison, A.; Anderson, H.; et al. Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2270–2290. [Google Scholar] [CrossRef] [PubMed]
- Copelan, E.A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006, 354, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Thierer, T.; Bitan, M.; Shapira, M.Y.; Meyerowitz, C. A decision analysis: The dental management of patients prior to hematology cytotoxic therapy or hematopoietic stem cell transplantation. Oral Oncol. 2008, 44, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.M.; Bruce, A.J.; Wolf, R.C.; Litzow, M.R.; Hogan, W.J.; Patnaik, M.S.; Kremers, W.K.; Phillips, G.L.; Hashmi, S.K. The Incidence and Severity of Oral Mucositis among Allogeneic Hematopoietic Stem Cell Transplantation Patients: A Systematic Review. Biol. Blood Marrow Transplant. 2016, 22, 605–616. [Google Scholar] [CrossRef]
- van Leeuwen, S.J.M.; Potting, C.M.J.; Huysmans, M.; Blijlevens, N.M.A. Salivary Changes before and after Hematopoietic Stem Cell Transplantation: A Systematic Review. Biol. Blood Marrow Transplant. 2019, 25, 1055–1061. [Google Scholar] [CrossRef]
- Baird, K.; Pavletic, S.Z. Chronic graft versus host disease. Curr. Opin. Hematol 2006, 13, 426–435. [Google Scholar] [CrossRef]
- Imanguli, M.M.; Alevizos, I.; Brown, R.; Pavletic, S.Z.; Atkinson, J.C. Oral graft-versus-host disease. Oral Dis. 2008, 14, 396–412. [Google Scholar] [CrossRef]
- Bustillos, H.; Indorf, A.; Alwan, L.; Thompson, J.; Jung, L. Xerostomia: An immunotherapy-related adverse effect in cancer patients. Support. Care Cancer 2022, 30, 1681–1687. [Google Scholar] [CrossRef]
- Lederhandler, M.H.; Ho, A.; Brinster, N.; Ho, R.S.; Liebman, T.N.; Lo Sicco, K. Severe Oral Mucositis: A Rare Adverse Event of Pembrolizumab. J. Drugs Dermatol. 2018, 17, 807–809. [Google Scholar]
- Owosho, A.A.; Randazzo, J.; Rosen, E.B.; Estilo, C.L.; Huryn, J.M.; Chi, P.; Yom, S.K. Squamous cell carcinoma associated with chronic graft versus host disease-like/lichen planus-like lesion of the oral cavity in a patient managed for metastatic melanoma with a PD-1 inhibitor pembrolizumab. Oral Oncol. 2016, 63, e1–e3. [Google Scholar] [CrossRef]
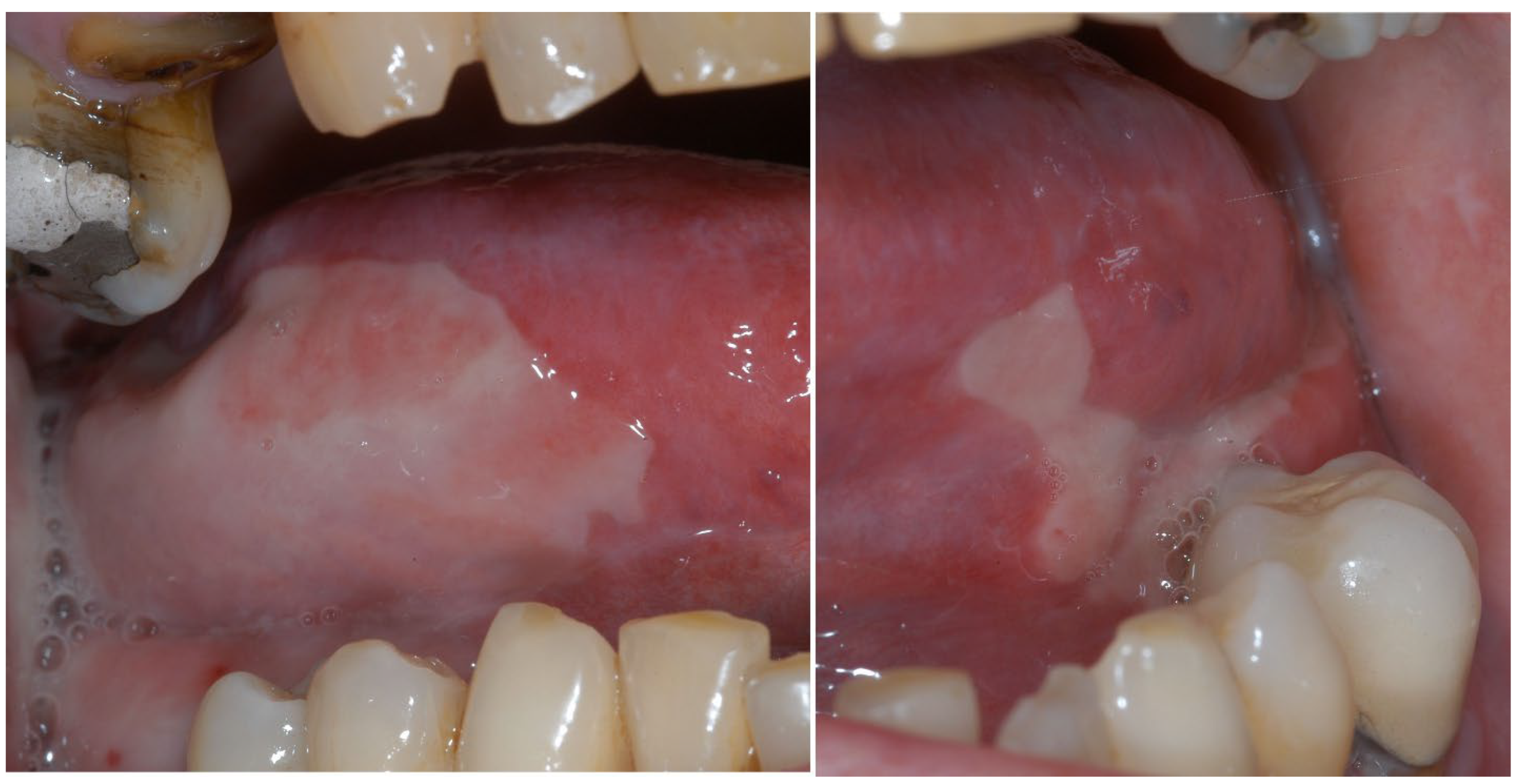
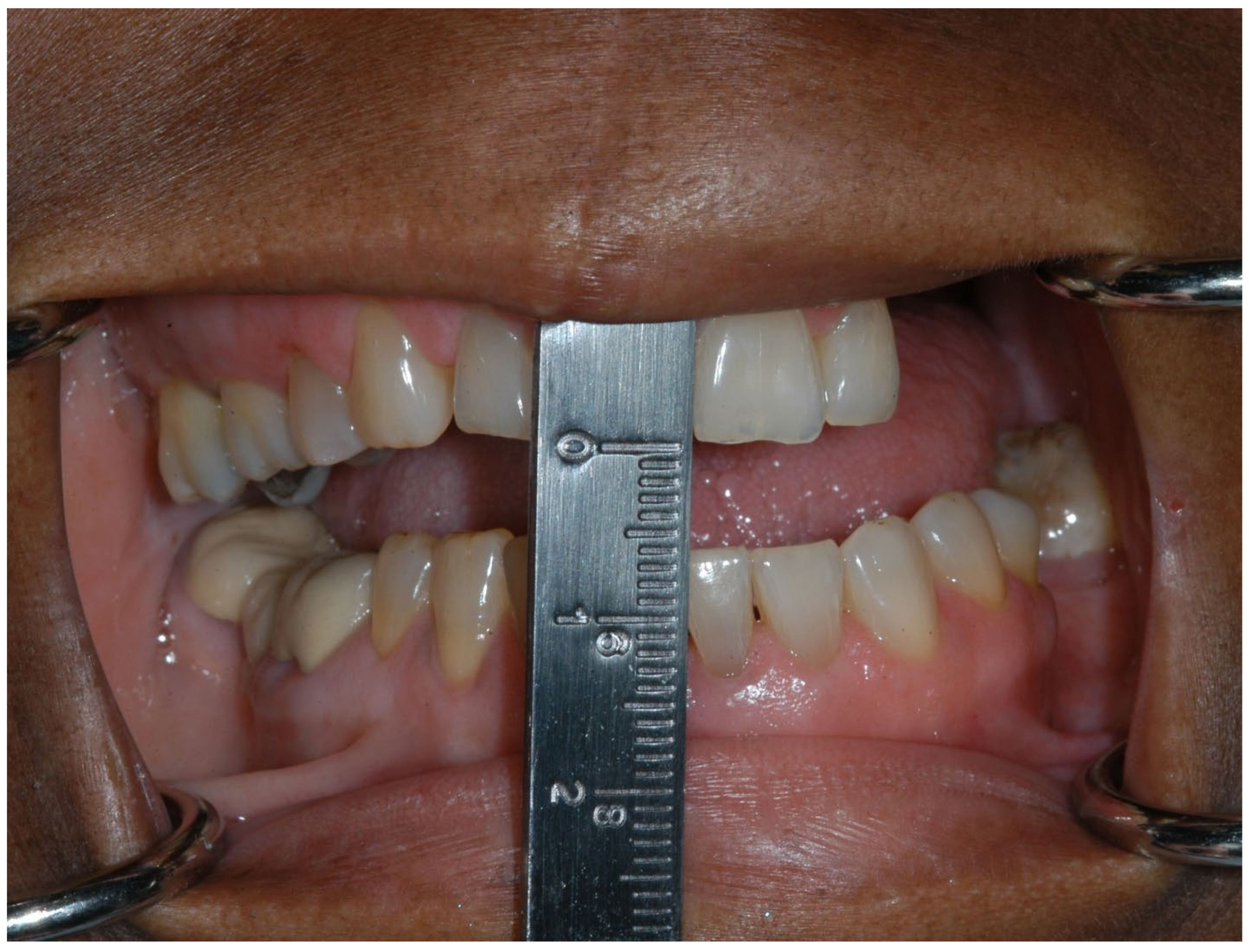
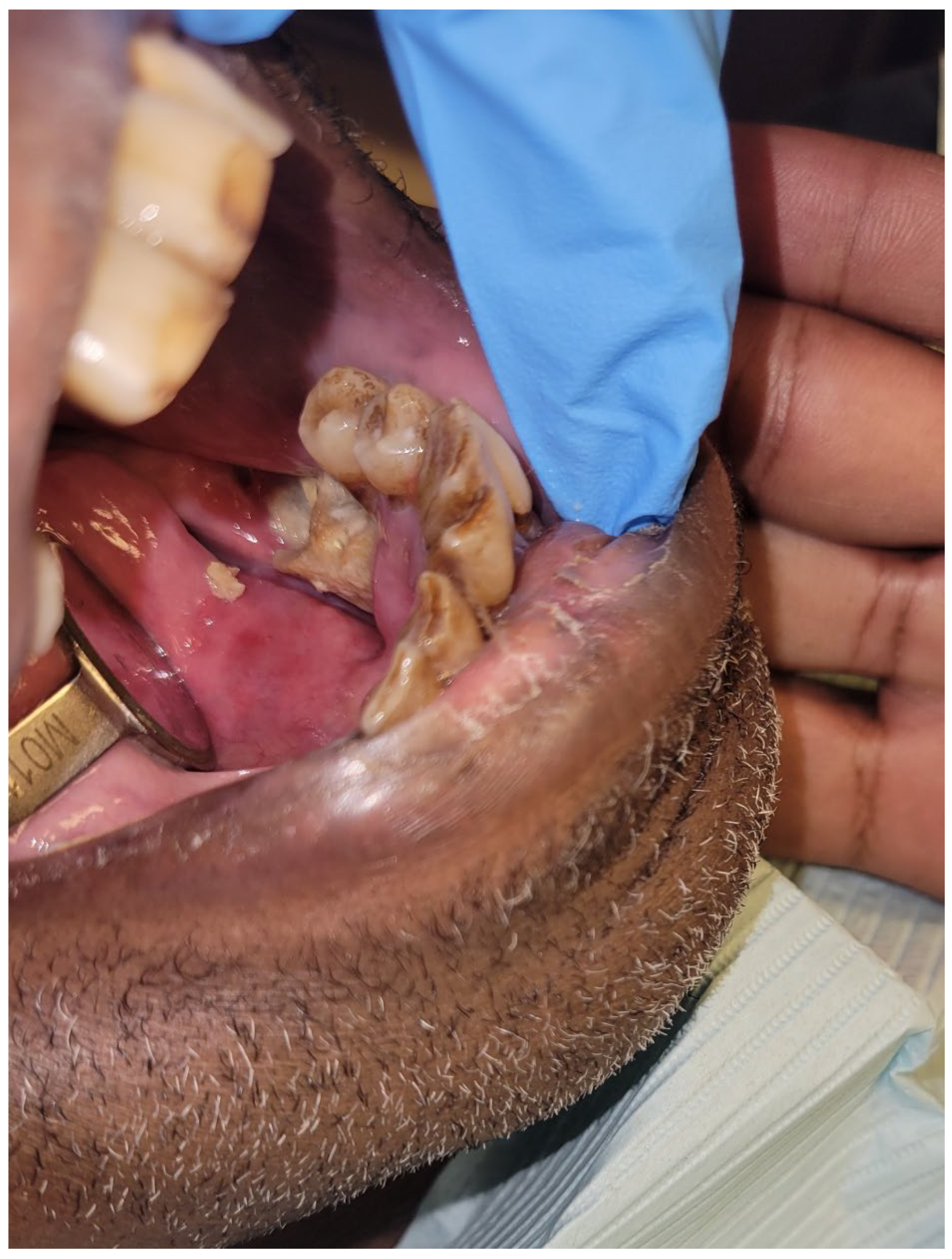
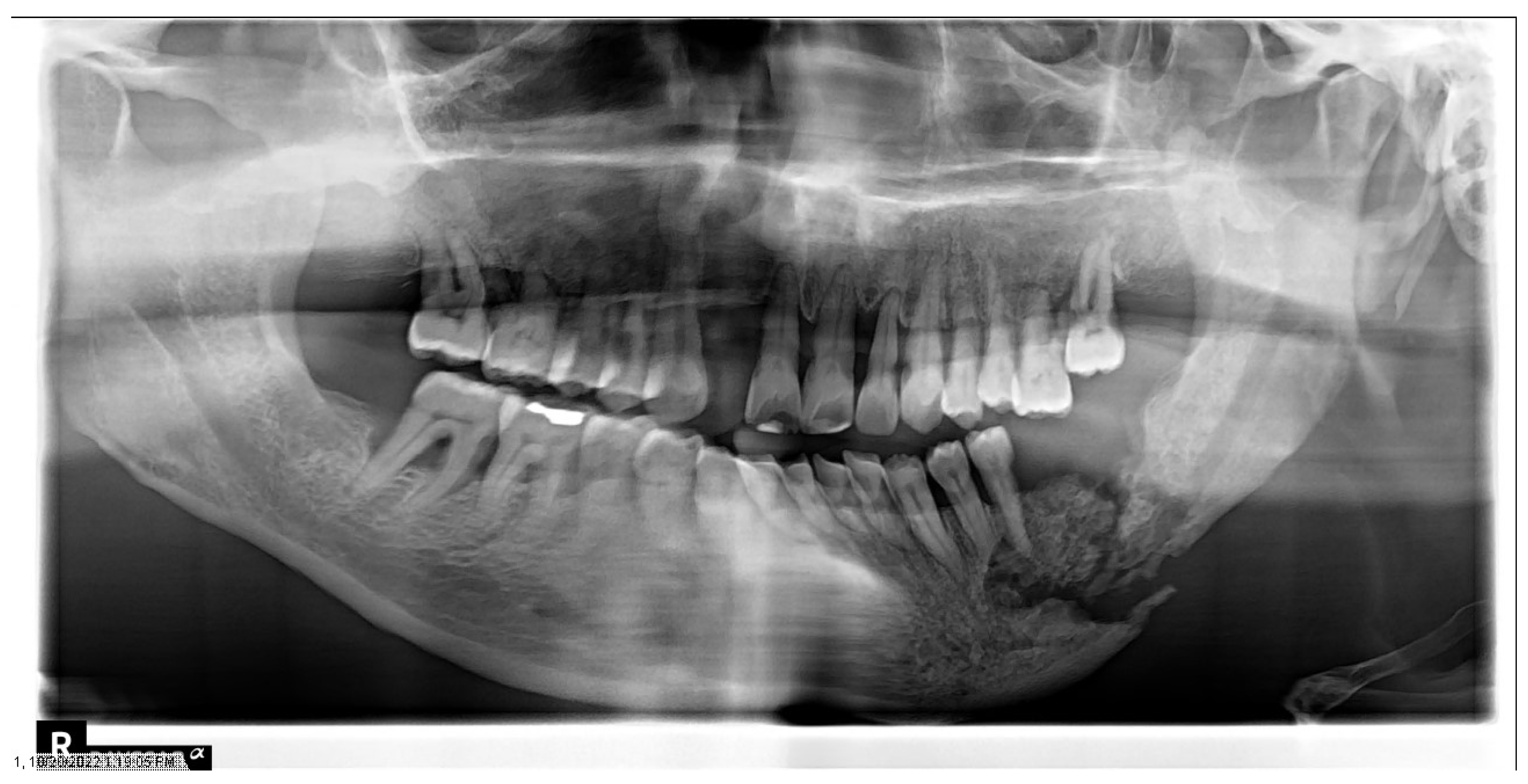
| Section | Guideline Statement |
|---|---|
| Basic oral care | The panel suggests that implementation of multiagent combination oral care protocols (MCOCP) is beneficial for the prevention of oral mucositis during chemotherapy, head and neck radiotherapy and hematopoietic stem cell transplant (HSCT). MCOCP—these protocols serve to increase the awareness of patients and staff of the importance of good oral hygiene that may lead to fewer and less severe oral complications; typically, the protocols involve recommendations about the timing, frequency and products used, which include various combinations of bland mouth rinses, toothbrushes and flossing procedures. |
| Anti-inflammatory agents | The panel recommends benzydamine mouthwash for the prevention of oral mucositis in patients with H and N cancer receiving a moderate-dose RT (<50 Gy). |
| The panel suggests the use of benzydamine mouthwash for the prevention of oral mucositis in patients with head and neck cancer who receive chemoradiation therapy. | |
| Photobiomodulation therapy | The panel recommends the use of intraoral photobiomodulation therapy using low-level laser therapy for the prevention of oral mucositis in adult patients receiving radiotherapy ± chemotherapy for head and neck cancers or HSCT conditioned with high-dose chemotherapy ± total body irradiation. Safety considerations unique to patients with oral cancer should be considered. |
| Cryotherapy | The panel recommends using oral cryotherapy to prevent oral mucositis in patients undergoing autologous HSCT when the conditioning includes high dose melphalan. |
| The panel recommends using 30 min of oral cryotherapy to prevent oral mucositis in patients receiving bolus 5-FU chemotherapy during the infusion. | |
| Antimicrobials, coating agents, anesthetics and analgesics | Topical morphine 0.2% mouthwash is suggested for the treatment of oral mucositis-associated pain in patients with head and neck cancer who receive chemoradiation therapy. |
| Growth factors and cytokines | The use of KGF-1 intravenously is recommended for the prevention of oral mucositis in patients with hematologic cancer undergoing autologous HSCT with a conditioning regimen that includes high-dose chemotherapy and total body irradiation. |
| Natural and miscellaneous | Honey is suggested for the prevention of oral mucositis in patients with head and neck cancer who receive chemoradiation therapy. |
| Clinical question 1. What is the efficacy of available pharmacologic and nonpharmacologic interventions (including the effects of radiation dose, type and regimen) for the prevention of xerostomia induced by nonsurgical cancer therapies? |
| Recommendation 1.1. IMRT should be used to spare major and minor salivary glands from a higher dose of radiation to reduce the risk of salivary gland hypofunction and xerostomia in patients with head and neck cancer (type: evidence-based; evidence quality: high; strength of recommendation: strong). |
| Recommendation 1.2. Other radiation modalities that limit cumulative dose to and irradiated volume of major and minor salivary glands as or more effectively than intensity-modulated radiation therapy may be offered to reduce salivary gland hypofunction and xerostomia (type: informal consensus; evidence quality: low; strength of recommendation: strong). |
| Recommendation 1.3. Acupuncture may be offered during radiation therapy for head and neck cancer to reduce the risk of developing xerostomia (type: evidence-based; evidence quality: intermediate; strength of recommendation: moderate). |
| Recommendation 1.4. Systemic administration of the sialogogue bethanechol may be offered during radiation therapy for head and neck cancer to reduce the risk of salivary gland hypofunction and xerostomia (type: evidence-based; evidence quality: low; strength of recommendation: weak). |
| Clinical question 2. What is the efficacy of available pharmacologic and nonpharmacologic interventions for the management of xerostomia induced by nonsurgical cancer therapies? |
| Recommendation 2.1. Topical mucosal lubricants or saliva substitutes (agents directed at ameliorating xerostomia and other salivary gland hypofunction-related symptoms) may be offered to improve xerostomia induced by nonsurgical cancer therapies (type: evidence-based; evidence quality: intermediate; strength of recommendation: strong). |
| Recommendation 2.2. Gustatory and masticatory salivary reflex stimulation by sugar-free lozenges, acidic (nonerosive and sugar-free special preparation if dentate patients) candies or sugar-free, nonacidic chewing gum may be offered to produce transitory increased saliva flow rate and transitory relief from xerostomia by stimulating residual capacity of salivary gland tissue (type: evidence-based; evidence quality: intermediate; strength of recommendation: moderate). |
| Recommendation 2.3. Oral pilocarpine, and cevimeline where available, may be offered after radiation therapy in patients with head and neck cancer for transitory improvement of xerostomia and salivary gland hypofunction by stimulating residual capacity of salivary gland tissue. However, improvement of salivary gland hypofunction may be limited (type: evidence-based; evidence quality: high; strength of recommendation: strong). |
| Recommendation 2.4. Acupuncture may be offered after radiation therapy in patients with head and neck cancer for improvement of xerostomia (type: evidence-based; evidence quality: low; strength of recommendation: weak). |
| Recommendation 2.5. Transcutaneous electrostimulation or acupuncture-like transcutaneous electrostimulation of the salivary glands may be offered after radiation therapy in patients with head and neck cancer for improvement of salivary gland hypofunction and xerostomia (type: evidence-based; evidence quality: low; strength of recommendation: weak) |
| Clinical Question 2. What steps should be taken to reduce the risk of MRONJ? |
| Recommendation 2.1: Coordination of care: for patients with cancer who are scheduled to receive a BMA in a nonurgent setting, oral care assessment (including a comprehensive dental, periodontal and oral radiographic exam when feasible to do so) should be undertaken before initiating therapy. Based on the assessment, a dental care plan should be developed and implemented. The care plan should be coordinated between the dentist and the oncologist to ensure that medically necessary dental procedures are undertaken before the initiation of the BMA. Follow-up by the dentist should then be performed on a routine schedule, such as every 6 months once therapy with a BMA has commenced (Type: evidence based; Evidence quality: low/intermediate; Strength of recommendation: moderate). |
| Recommendation 2.2. Modifiable risk factors: members of the multidisciplinary team should address modifiable risk factors for MRONJ with the patient as early as possible. These risk factors include poor oral health, invasive dental procedures, ill-fitting dentures, uncontrolled diabetes mellitus and tobacco use (Type: formal consensus; Evidence quality: insufficient; Strength of recommendation: moderate). |
| Recommendation 2.3. Elective dentoalveolar surgery: elective dentoalveolar surgical procedures (e.g., nonmedically necessary extractions, alveoloplasties and implants) should not be performed during active therapy with a BMA at an oncologic dose. Exceptions may be considered when a dental specialist with expertise in the prevention and treatment of MRONJ has reviewed the benefits and risks of the proposed invasive procedure with the patient and the oncology team (Type: evidence based; Evidence quality: intermediate; Strength of recommendation: moderate). |
| Recommendation 2.4. Dentoalveolar surgery follow-up: if dentoalveolar surgery is performed, patients should be evaluated by the dental specialist on a systematic and frequently scheduled basis (e.g., every 6 to 8 weeks) until full mucosal coverage of the surgical site has occurred. Communication with the oncologist regarding the status of healing is encouraged, particularly when considering future use of BMA (Type: formal consensus; Evidence quality: insufficient; Strength of recommendation: moderate). |
| Recommendation 2.5. Temporary discontinuation of BMAs before dentoalveolar surgery: for patients with cancer who are receiving a BMA at an oncologic dose, there is insufficient evidence to support or refute the need for discontinuation of the BMA before dentoalveolar surgery. Administration of the BMA may be deferred at the discretion of the treating physician, in conjunction with discussion with the patient and the oral health provider (Type: informal consensus; Evidence quality: insufficient; Strength of recommendation: weak). |
| Clinical Question 4. How should MRONJ be managed? |
| Recommendation 4.1: Initial treatment of MRONJ: conservative measures comprise the initial approach to treatment of MRONJ. Conservative measures may include antimicrobial mouth rinses, antibiotics if clinically indicated, effective oral hygiene and conservative surgical interventions, such as removal of a superficial bone spicule (Type: formal consensus; Evidence quality: insufficient; Strength of recommendation: moderate). |
| Recommendation 4.2: Treatment of refractory MRONJ: aggressive surgical interventions (e.g., mucosal flap elevation, block resection of necrotic bone or soft tissue closure) may be used if MRONJ results in persistent symptoms or affects function despite initial conservative treatment. Aggressive surgical intervention is not recommended for asymptomatic bone exposure. In advance of the aggressive surgical intervention, the multidisciplinary care team and patient should thoroughly discuss the risks and benefits of the proposed intervention (Type: formal consensus; Evidence quality: insufficient; Strength of recommendation: weak). |
| Clinical Question 5. Should BMAs be temporarily discontinued after a diagnosis of MRONJ has been made? |
| Recommendation 5. For patients who are diagnosed with MRONJ while being treated with BMAs, there is insufficient evidence to support or refute the discontinuation of the BMAs. Administration of the BMA may be deferred at the discretion of the treating physician, in conjunction with discussion with the patient and the oral health provider (Type: formal consensus; Evidence quality: insufficient; Strength of recommendation: weak). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owosho, A.A.; DeColibus, K.; Hedgepeth, B.; Wood, B.C.; Sansoni, R.E.; Gleysteen, J.P.; Schwartz, D.L. The Role of Dental Practitioners in the Management of Oncology Patients: The Head and Neck Radiation Oncology Patient and the Medical Oncology Patient. Dent. J. 2023, 11, 136. https://doi.org/10.3390/dj11050136
Owosho AA, DeColibus K, Hedgepeth B, Wood BC, Sansoni RE, Gleysteen JP, Schwartz DL. The Role of Dental Practitioners in the Management of Oncology Patients: The Head and Neck Radiation Oncology Patient and the Medical Oncology Patient. Dentistry Journal. 2023; 11(5):136. https://doi.org/10.3390/dj11050136
Chicago/Turabian StyleOwosho, Adepitan A., Katherine DeColibus, Beverly Hedgepeth, Burton C. Wood, Ritter E. Sansoni, John P. Gleysteen, and David L. Schwartz. 2023. "The Role of Dental Practitioners in the Management of Oncology Patients: The Head and Neck Radiation Oncology Patient and the Medical Oncology Patient" Dentistry Journal 11, no. 5: 136. https://doi.org/10.3390/dj11050136
APA StyleOwosho, A. A., DeColibus, K., Hedgepeth, B., Wood, B. C., Sansoni, R. E., Gleysteen, J. P., & Schwartz, D. L. (2023). The Role of Dental Practitioners in the Management of Oncology Patients: The Head and Neck Radiation Oncology Patient and the Medical Oncology Patient. Dentistry Journal, 11(5), 136. https://doi.org/10.3390/dj11050136








