Management of Medication-Related Osteonecrosis of the Jaw with Photobiomodulation and Minimal Surgical Intervention
Abstract
1. Introduction
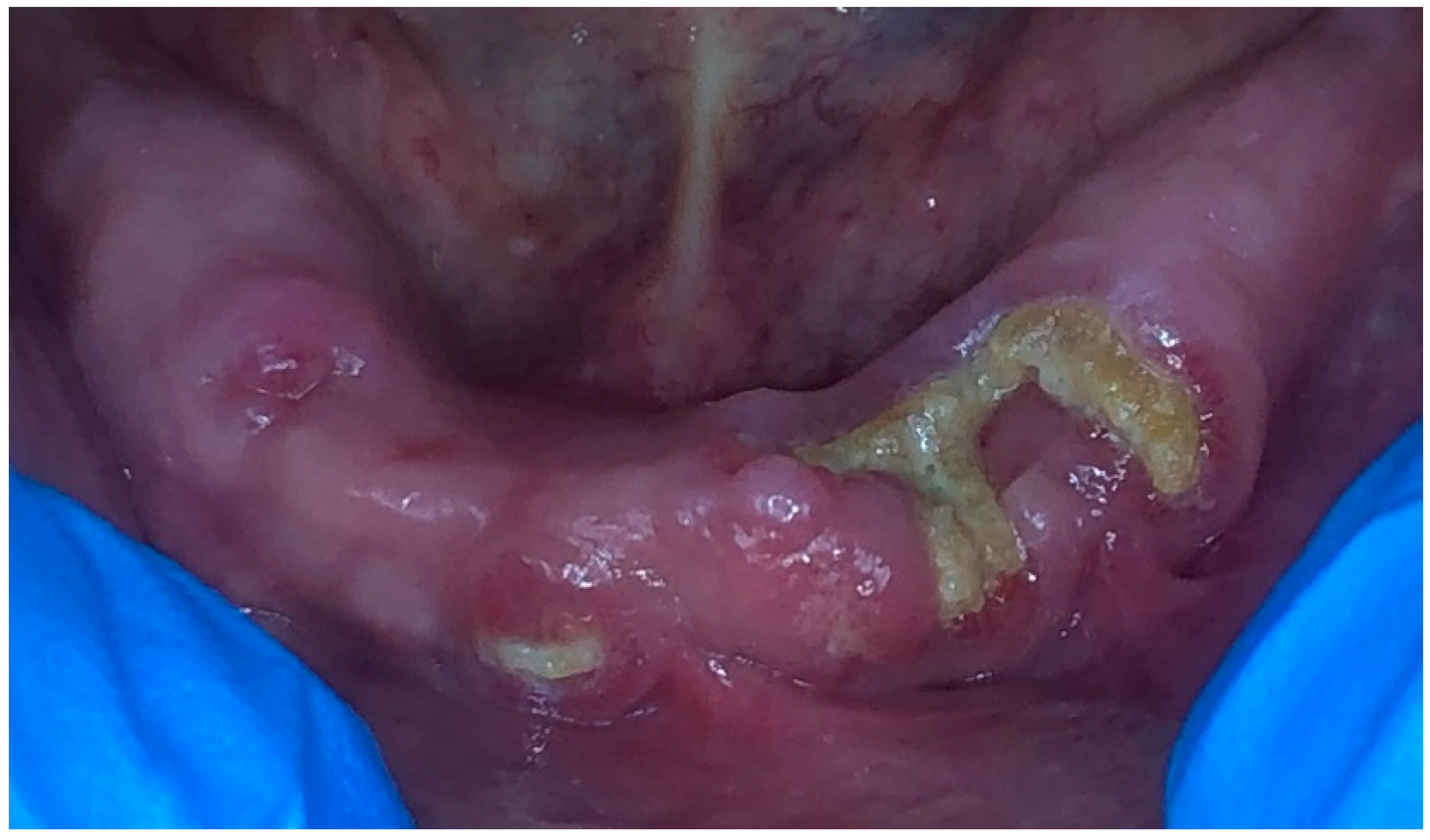
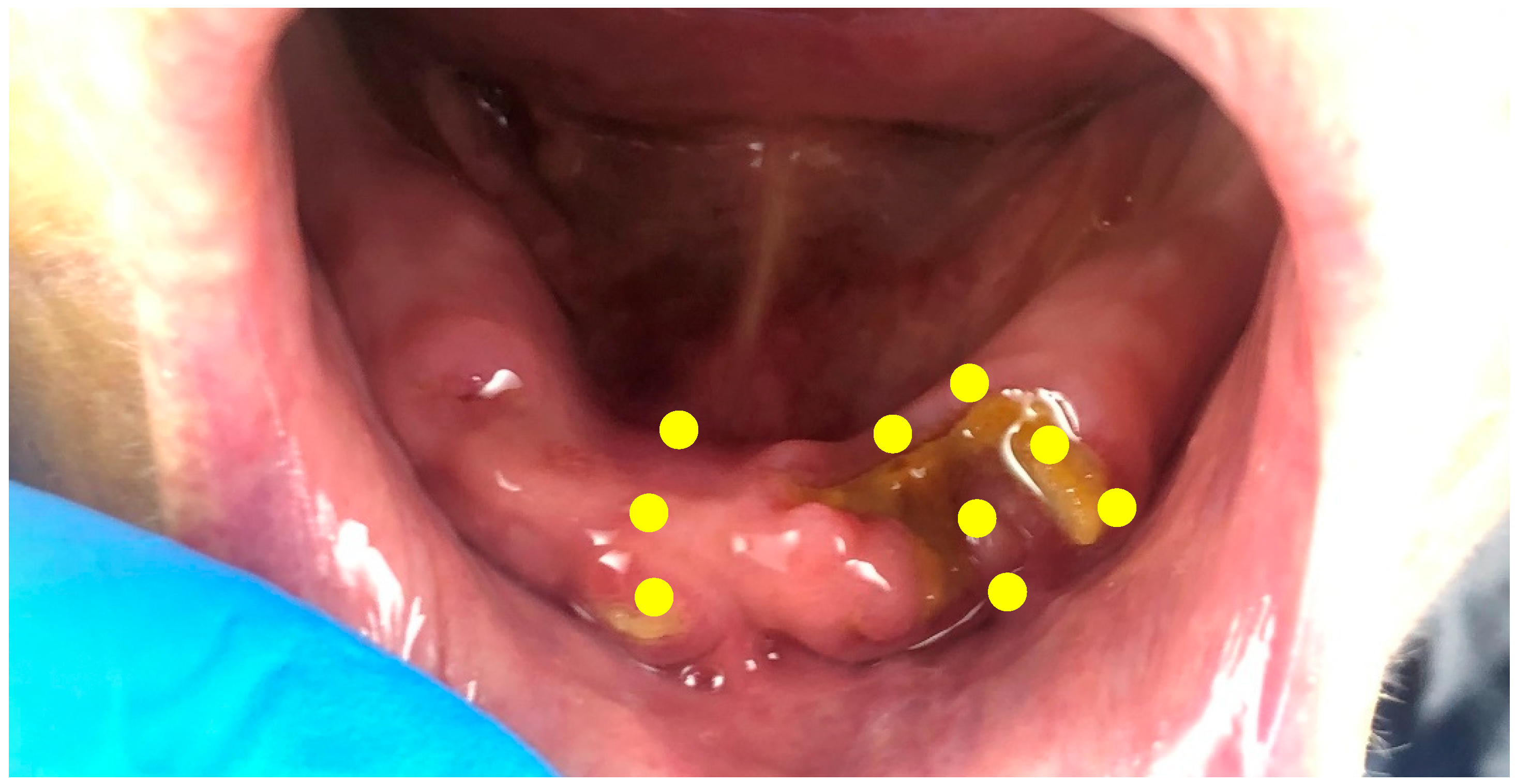
2. Case Presentation
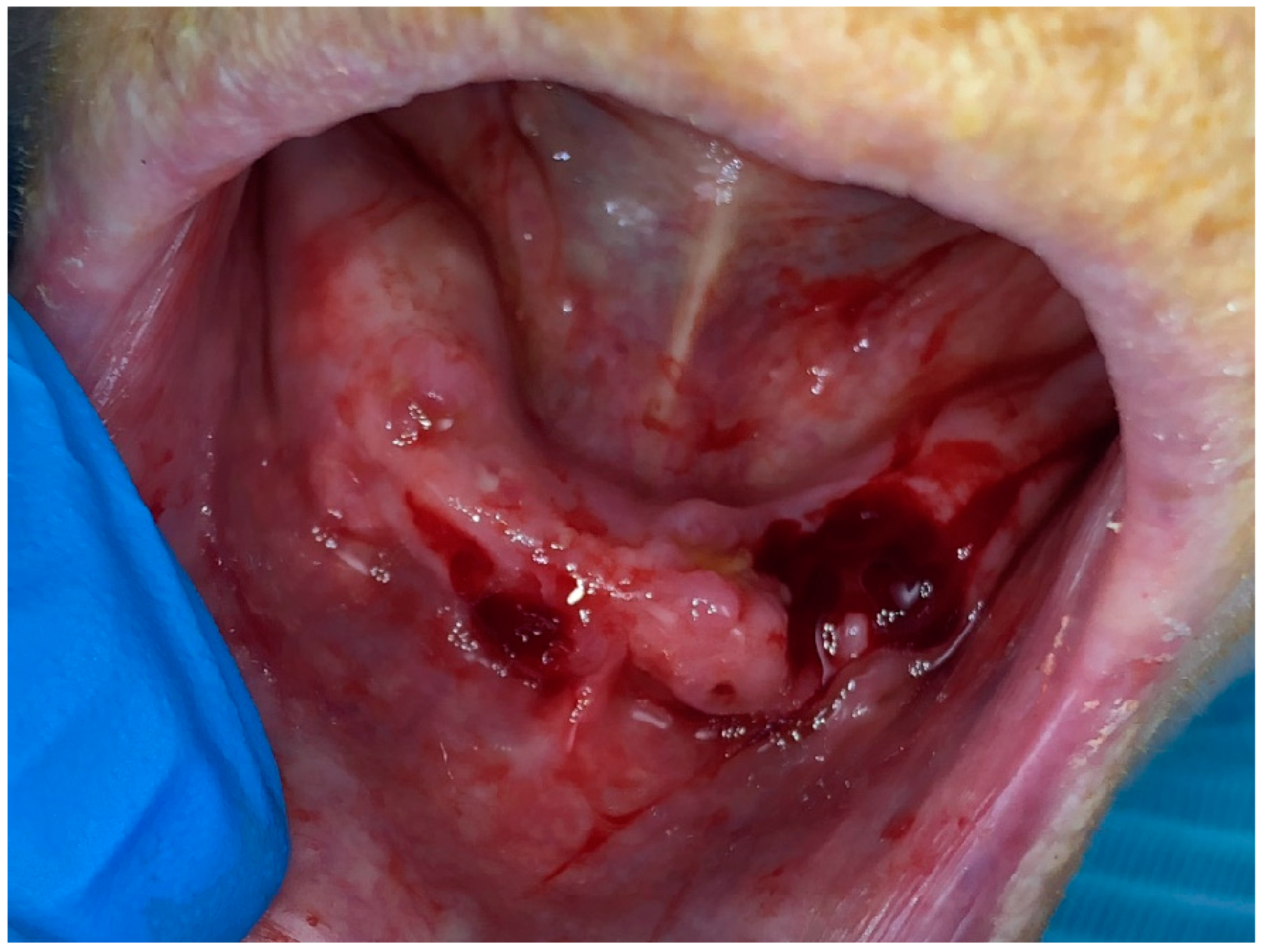
2.1. Treatment Protocol and Instructions
2.1.1. Photobiomodulation Therapy
2.1.2. Surgical Intervention
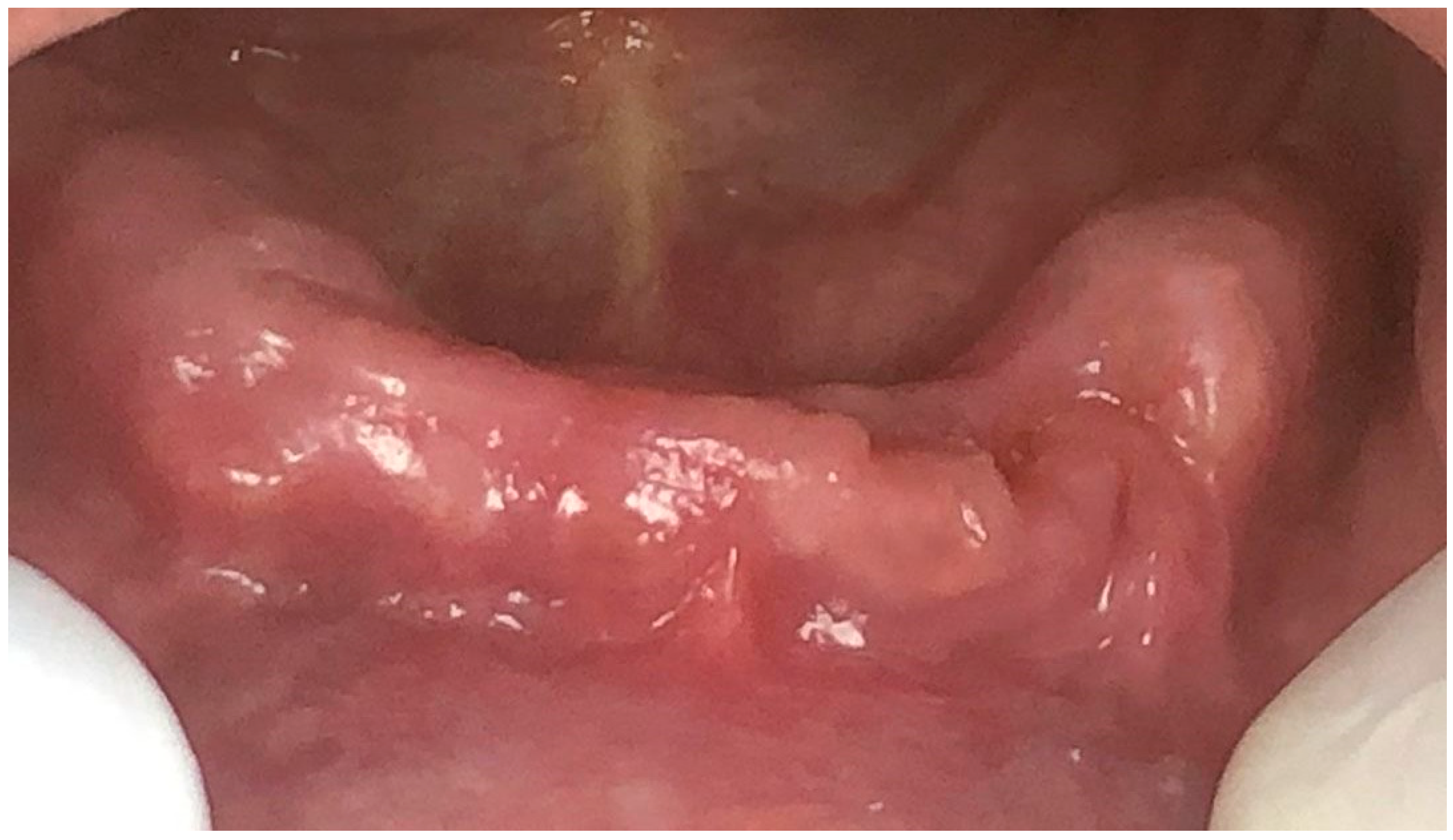
2.2. Result
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- AlDhalaan, N.A.; BaQais, A.; Al-Omar, A. Medication-related osteonecrosis of the jaw: A review. Cureus 2020, 12, e6944. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, I.S.; Ngwa, B.A.; Gong, Y. Drug induced osteonecrosis of the jaw. Cancer Treat. Rev. 2015, 41, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Alsalih, A.; Dam, A.; Lindberg, P.; Truedsson, A. Medication-related osteonecrosis of the jaws initiated by zoledronic acid and potential pathophysiology. Dent. J. 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, T. Pathophysiology of BRONJ: Drug-related osteoclastic disease of the jaw. Oral Sci. Int. 2013, 10, 1–8. [Google Scholar] [CrossRef]
- Rosella, D.; Papi, P.; Giardino, R.; Cicalini, E.; Piccoli, L.; Pompa, G. Medication-related osteonecrosis of the jaw: Clinical and practical guidelines. J. Int. Soc. Prev. Community Dent. 2016, 6, 97. [Google Scholar]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef]
- Heufelder, M.J.; Hendricks, J.; Remmerbach, T.; Frerich, B.; Hemprich, A.; Wilde, F. Principles of oral surgery for prevention of bisphosphonate-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 429–435. [Google Scholar] [CrossRef]
- Weitzman, R.; Sauter, N.; Eriksen, E.F.; Tarassoff, P.G.; Lacerna, L.V.; Dias, R.; Altmeyer, A.; Csermak-Renner, K.; McGrath, L.; Lantwicki, L.; et al. Critical review: Updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients 2006. Crit. Rev. Oncol./Hematol. 2007, 62, 148–152. [Google Scholar] [CrossRef]
- Hoefert, S.; Eufinger, H. Relevance of a prolonged preoperative antibiotic regime in the treatment of bisphosphonate-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg. 2011, 69, 362–380. [Google Scholar] [CrossRef]
- Sacco, R.; Leeson, R.; Nissan, J.; Olate, S.; Bettoni Cruz de Castro, C.H.; Acocella, A.; Lalli, A. A systematic review of oxygen therapy for the management of medication-related osteonecrosis of the jaw (MRONJ). Appl. Sci. 2019, 9, 1026. [Google Scholar] [CrossRef]
- Vanpoecke, J.; Verstraete, L.; Smeets, M.; Ferri, J.; Nicot, R.; Politis, C. Medication-related osteonecrosis of the jaw (MRONJ) stage III: Conservative and conservative surgical approaches versus an aggressive surgical intervention: A systematic review. J. Cranio-Maxillofac. Surg. 2020, 48, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.B.; Camilotti, R.S.; Ponte, M.E. Efficacy of laser therapy in the management of bisphosphonate-related osteonecrosis of the jaw (BRONJ): A systematic review. Lasers Med. Sci. 2016, 31, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Razavi, P.; Jafari, A.; Vescovi, P.; Fekrazad, R. Efficacy of Adjunctive Photobiomodulation in the Management of Medication-Related Osteonecrosis of the Jaw: A Systematic Review. Photobiomodul. Photomed. Laser Surg. 2022, 40, 777–791. [Google Scholar] [CrossRef]
- Gruppo, R.; Glueck, C.J.; Mcmahon, R.E.; Bouquot, J.; Rabinovich, B.A.; Becker, A.; Tracy, T.; Wang, P. The pathophysiology of alveolar osteonecrosis of the jaw: Anticardiolipin antibodies, thrombophilia, and hypofibrinolysis. J. Lab. Clin. Med. 1996, 127, 481–488. [Google Scholar] [CrossRef]
- Ma, J.X.; Yang, Q.M.; Xia, Y.C.; Zhang, W.G.; Nie, F.F. Effect of 810 nm near-infrared laser on revascularization of ischemic flaps in rats. Photomed. Laser Surg. 2018, 36, 290–297. [Google Scholar] [CrossRef]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 1: Mechanisms of action, dosimetric, and safety considerations. Support. Care Cancer 2016, 24, 2781–2792. [Google Scholar] [CrossRef]
- El Mobadder, M.; Farhat, F.; El Mobadder, W.; Nammour, S. Photobiomodulation therapy in the treatment of oral mucositis, dysphagia, oral dryness, taste alteration, and burning mouth sensation due to cancer therapy: A case series. Int. J. Environ. Res. Public Health 2019, 16, 4505. [Google Scholar] [CrossRef]
- Berni, M.; Brancato, A.M.; Torriani, C.; Bina, V.; Annunziata, S.; Cornella, E.; Trucchi, M.; Jannelli, E.; Mosconi, M.; Gastaldi, G.; et al. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. Int. J. Mol. Sci. 2023, 24, 7094. [Google Scholar] [CrossRef]
- Mester, S.; Spiry, R.; Szende, L.; Tota, G. Infrared laser therapy in wound healing. Am. J. Phys. Med. 1967, 46, 93–103. [Google Scholar]
- Srivastava, S.; Ebenezer, V.; Balakrishnan, R. Lasers in Oral and Maxillofacial Surgery—A Review. J. Pharm. Sci. Res. 2023, 15, 1057–1059. [Google Scholar]
- Chen, A.C.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, 22453. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Moghaddam, A.S.; Manouchehri, S.; Atoufi, Z.; Amiri, A.; Amirkhani, M.A.; Nilforoushzadeh, M.A.; Saeb, M.R.; Hamblin, M.R.; Mozafari, M. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine 2017, 12, 2403–2422. [Google Scholar] [CrossRef] [PubMed]
- Tumilty, S.; Munn, J.; McDonough, S.; Hurley, D.A.; Basford, J.R.; Baxter, G.D. Low level laser treatment of tendinopathy: A systematic review with meta-analysis. Photomed. Laser Surg. 2010, 28, 3–16. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Hamblin, M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Kulkarni, S.; Meer, M.; George, R. Efficacy of photobiomodulation on accelerating bone healing after tooth extraction: A systematic review. Lasers Med. Sci. 2019, 34, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.D.; Limirio, J.P.; Zanatta, L.S.; Simamoto, V.R.; Dechichi, P.; Limirio, A.P. Effectiveness of photobiomodulation therapy on human bone healing in dentistry: A systematic review. Photobiomodul. Photomed. Laser Surg. 2022, 40, 440–453. [Google Scholar] [CrossRef]
- Marx, R.E. Oral and Intravenous Bisphosphonate-Induced Osteonecrosis of the Jaws; Quintessence: Chicago, IL, USA, 2007. [Google Scholar]
- Jamalpour, M.R.; Shahabi, S.; Baghestani, M.; Shokri, A.; Jamshidi, S.; Khazaei, S. Complementarity of surgical therapy, photobiomodulation, A-PRF and L-PRF for management of medication-related osteonecrosis of the jaw (MRONJ): An animal study. BMC Oral Health 2022, 22, 241. [Google Scholar] [CrossRef]
- Roato, I.; Mauceri, R.; Notaro, V.; Genova, T.; Fusco, V.; Mussano, F. Immune Dysfunction in Medication-Related Osteonecrosis of the Jaw. Int. J. Mol. Sci. 2023, 24, 7948. [Google Scholar] [CrossRef]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Min. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef]
- Okuyama, K.; Hayashida, S.; Rokutanda, S.; Kawakita, A.; Soutome, S.; Sawada, S.; Yanamoto, S.; Kojima, Y.; Umeda, M. Surgical strategy for medication-related osteonecrosis of the jaw (MRONJ) on maxilla: A multicenter retrospective study. J. Dent. Sci. 2021, 16, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Fliefel, R.; Troltzsch, M.; Kuhnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef] [PubMed]
- El-Rabbany, M.; Sgro, A.; Lam, D.K.; Shah, P.S.; Azarpazhooh, A. Effectiveness of treatments for medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2017, 148, 584–594. [Google Scholar] [CrossRef]
- Li, F.L.; Wu, C.B.; Sun, H.J.; Zhou, Q. Effectiveness of laser-assisted treatments for medication-related osteonecrosis of the jaw: A systematic review. Br. J. Oral Maxillofac. Surg. 2020, 58, 256–267. [Google Scholar] [CrossRef]
- Schipmann, S.; Metzler, P.; Rössle, M.; Zemann, W.; von Jackowski, J.; Obwegeser, J.A.; Grätz, K.W.; Jacobsen, C. Osteopathology associated with bone resorption inhibitors–which role does Actinomyces play? A presentation of 51 cases with systematic review of the literature. J. Oral Pathol. Med. 2013, 42, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, J.; Zavodnik, L.; Zavodnik, I.; Buko, V.; Lapshyna, A.; Bryszewska, M. Effect of low-intensity (3.75–25 J/cm2) near-infrared (810 nm) laser radiation on red blood cell ATPase activities and membrane structure. J. Clin. Laser Med. Surg. 2004, 22, 111–117. [Google Scholar] [CrossRef]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 2: Mechanisms of action, dosimetric, and safety considerations. Support. Care Cancer 2016, 24, 2781–2792. [Google Scholar] [CrossRef]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; Genot, M.T.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 2: Proposed applications and treatment protocols. Support. Care Cancer 2016, 24, 2793–2805. [Google Scholar] [CrossRef]
| Stage | Description | Treatment |
|---|---|---|
| At risk |
|
|
| Stage 0 |
|
|
| Stage 1 |
|
|
| Stage 2 |
|
|
| Stage 3 | Exposed and necrotic bone or fistulas that probe to bone with evidence of infection and at least 1 of the following:
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mobadder, M.; Grzech-Lesniak, Z.; El Mobadder, W.; Rifai, M.; Ghandour, M.; Nammour, S. Management of Medication-Related Osteonecrosis of the Jaw with Photobiomodulation and Minimal Surgical Intervention. Dent. J. 2023, 11, 127. https://doi.org/10.3390/dj11050127
El Mobadder M, Grzech-Lesniak Z, El Mobadder W, Rifai M, Ghandour M, Nammour S. Management of Medication-Related Osteonecrosis of the Jaw with Photobiomodulation and Minimal Surgical Intervention. Dentistry Journal. 2023; 11(5):127. https://doi.org/10.3390/dj11050127
Chicago/Turabian StyleEl Mobadder, Marwan, Zuzanna Grzech-Lesniak, Wassim El Mobadder, Mohamad Rifai, Maher Ghandour, and Samir Nammour. 2023. "Management of Medication-Related Osteonecrosis of the Jaw with Photobiomodulation and Minimal Surgical Intervention" Dentistry Journal 11, no. 5: 127. https://doi.org/10.3390/dj11050127
APA StyleEl Mobadder, M., Grzech-Lesniak, Z., El Mobadder, W., Rifai, M., Ghandour, M., & Nammour, S. (2023). Management of Medication-Related Osteonecrosis of the Jaw with Photobiomodulation and Minimal Surgical Intervention. Dentistry Journal, 11(5), 127. https://doi.org/10.3390/dj11050127








