Assessment of the Periodontal Cementum Ablation Depth during Root Planing by an Er:YAG Laser at Different Energy Densities: An Ex Vivo Study
Abstract
1. Introduction
2. Materials and Methods
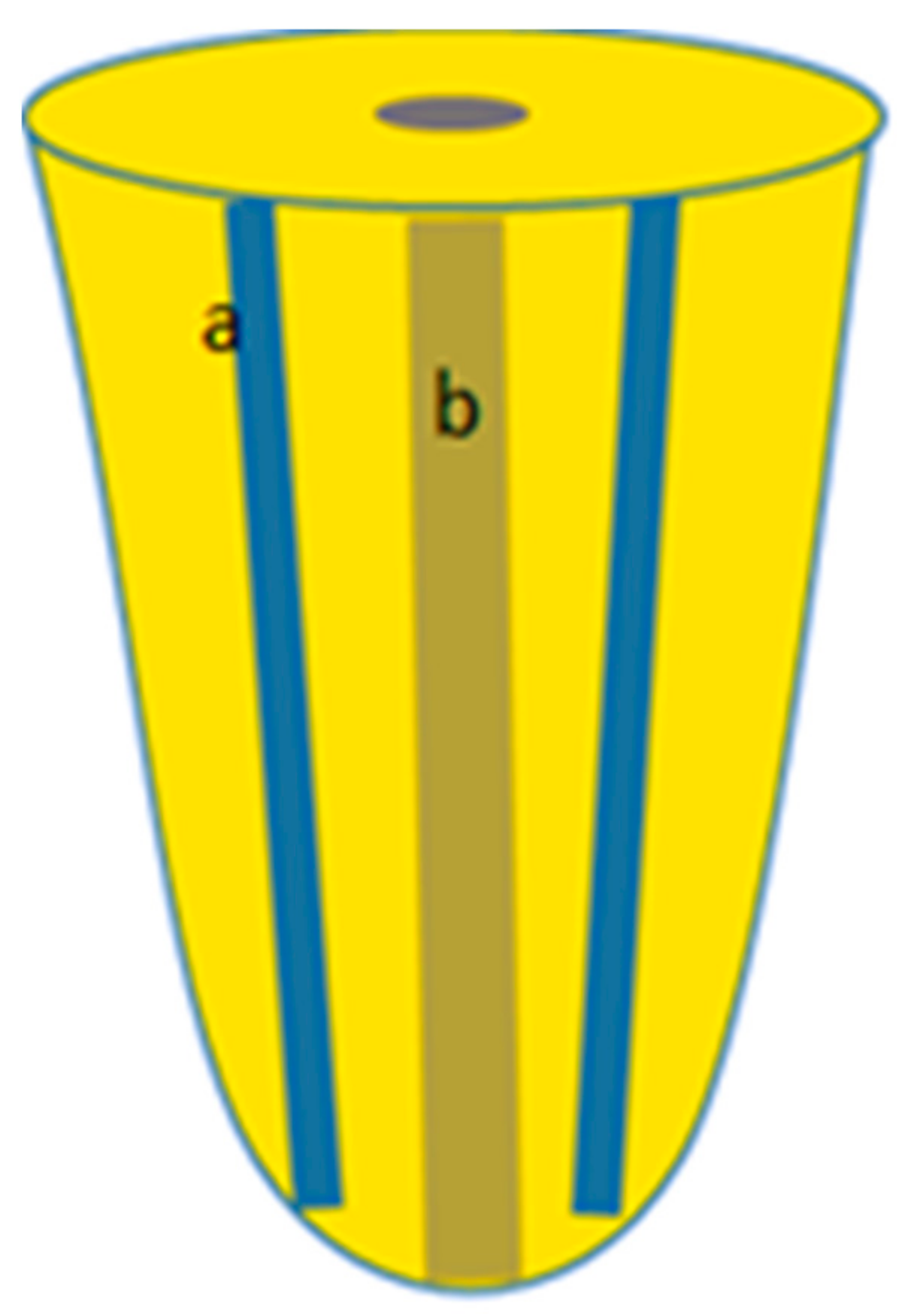
2.1. Samples Preparation
2.2. Samples Irradiation
2.3. Microscopic Observation
2.4. Statistical Analysis
3. Results
3.1. Optical Microscopic Observation
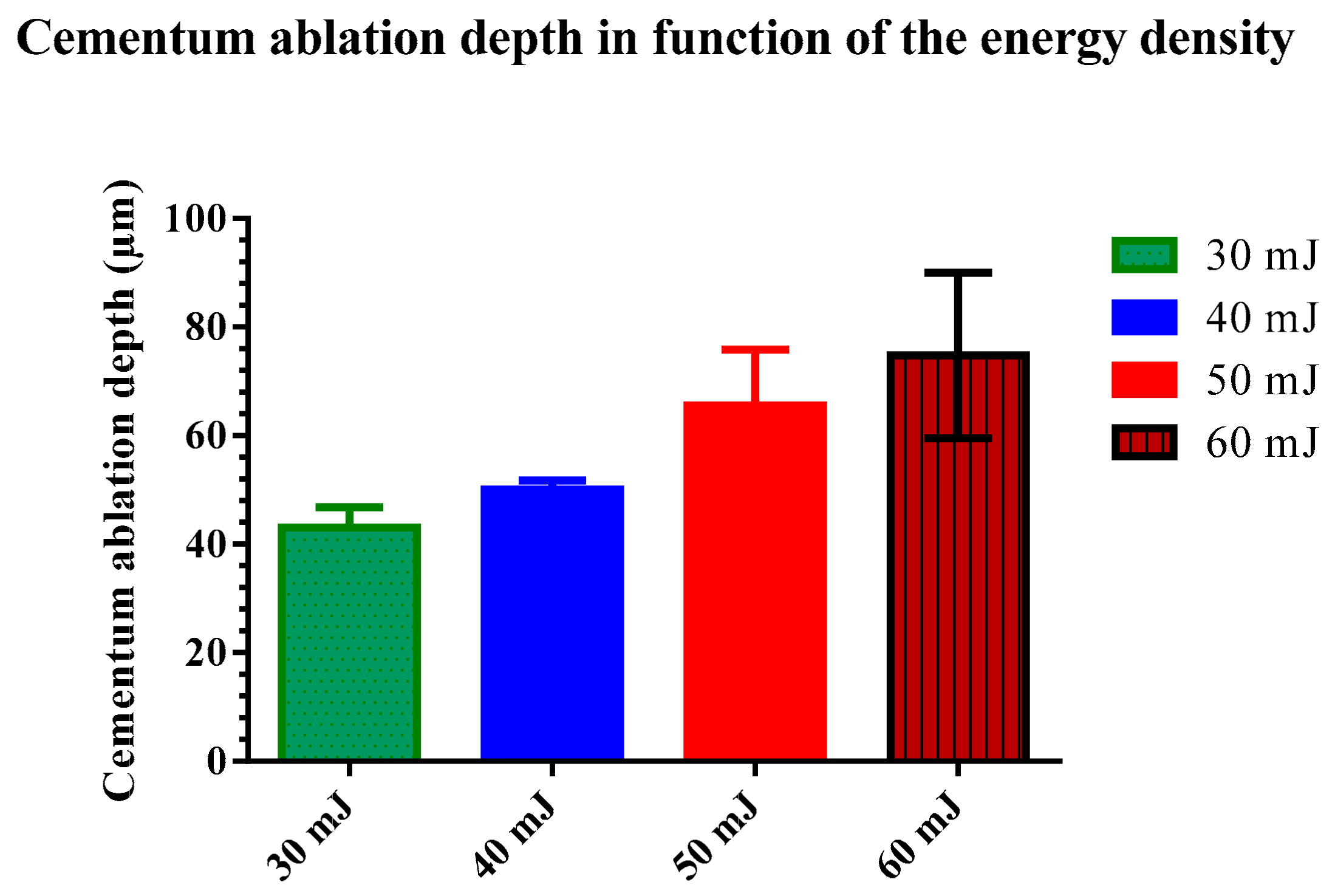
3.2. Statistical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shklar, G.; Carranza, F. Introduction: The Historical Background of Periodontology. In Newman and Carranza’s Clinical Periodontology, 13th ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 85–112. [Google Scholar]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Glickman, I. (Ed.) The scaling and curettage technique for the eradication of the periodontal pocket. In Clinical Periodontology; WB Saunders: Philadelphia, PA, USA, 1953; pp. 716–724. [Google Scholar]
- Pattison, A.; Pattison, G. Scaling and root planing. In Carranza’s Clinical Periodontology, 11th ed.; Newmann, M., Takei, H., Klokkevold, P., Carranza, F., Eds.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 461–473. [Google Scholar]
- Deas, D.E.; Moritz, A.J.; Sagun, R.S.; Gruwell, S.F., Jr.; Powell, C.A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol. 2000 2016, 71, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.P.; Kim, D.; Chang, Y.C. Anatomy, Structure, and Function of the Periodontium. In Clinical Periodontology, 13th ed.; Newmann, M., Takasaki, A.A., Klokkevold, P., Carranza, F., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 19–49. [Google Scholar]
- Stamfelj, I.; Vidmar, G.; Cvetko, E.; Gaspersic, D. Cementum thickness in multirooted human molars: A histometric study by light microscopy. Ann. Anat.-Anat. Anz. 2008, 190, 129–139. [Google Scholar] [CrossRef]
- George, M.; Donley, T.; Preshaw, P. Ultrasonic Periodontal Debridement, Theory and Technique; Wiley Blackwell: Oxford, UK, 2014. [Google Scholar]
- Saygin, N.E.; Giannobile, W.V.; Somerman, M.J. Molecular and cell biology of cementum. Periodontol. 2000 2000, 24, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Lamont, T.; Worthington, H.V.; Clarkson, J.E.; Beirne, P.V. Routine scale and polish for periodontal health in adults. Cochrane Database Syst. Rev. 2018, 12, Cd004625. [Google Scholar] [CrossRef]
- Megally, A.; Zekeridou, A.; Cancela, J.; Giannopoulou, C.; Mombelli, A. Short ultrasonic debridement with adjunctive low-concentrated hypochlorite/amino acid gel during periodontal maintenance: Randomized clinical trial of 12 months. Clin. Oral Investig. 2020, 24, 201–209. [Google Scholar] [CrossRef]
- Mlachkova, A.M.; Popova, C.L. Efficiency of nonsurgical periodontal therapy in moderate chronic periodontitis. Folia Med. 2014, 56, 109–115. [Google Scholar] [CrossRef]
- Aimetti, M. Nonsurgical periodontal treatment. Int. J. Esthet. Dent. 2014, 9, 251–267. [Google Scholar]
- Theodoro, L.; Garcia, V. Surgical and non-surgical treatment of periodontal diseases. In Lasers in Dentistry: Guide for Clinical Practice; Freitas, P., Simões, A., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 153–158. [Google Scholar]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Pesevska, S.; Gjorgoski, I.; Ivanovski, K.; Soldatos, N.K.; Angelov, N. The effect of low-level diode laser on COX-2 gene expression in chronic periodontitis patients. Lasers Med. Sci. 2017, 32, 1463–1468. [Google Scholar] [CrossRef]
- Malmström, H.S.; McCormack, S.M.; Fried, D.; Featherstone, J.D. Effect of CO2 laser on pulpal temperature and surface morphology: An in vitro study. J. Dent. 2001, 29, 521–529. [Google Scholar] [CrossRef]
- Laxman, K.V.; Ghosh, S.; Dhingra, K.; Patil, R. Effect of Er:YAG or Nd:YAG Laser Exposure on Fluorosed and Non-Fluorosed Root Surfaces: An In Vitro Study. Laser Ther. 2015, 24, 93–101. [Google Scholar] [CrossRef]
- Fu, C.S.; Liu, R.S.; Luo, Y.; Ou, L.; Li, Y.C.; Zhang, X.H. Changes of cementum endotoxin levels in different teeth with periodontitis treated with root conditioning. Shanghai Kou Qiang Yi Xue Shanghai J. Stomatol. 2017, 26, 175–179. [Google Scholar]
- Lukac, N.; Suhovršnik, T.; Lukac, M.; Jezeršek, M. Ablation characteristics of quantum square pulse mode dental erbium laser. J. Biomed. Opt. 2016, 21, 15012. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol. 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Liu, C.M.; Shyu, Y.C.; Pei, S.C.; Lan, W.H.; Hou, L.T. In vitro effect of laser irradiation on cementum-bound endotoxin isolated from periodontally diseased roots. J. Periodontol. 2002, 73, 1260–1266. [Google Scholar] [CrossRef]
- Hosseinipour, Z.S.; Pirmoradian-najafabadi, M.; Shahabi, S. Relationship between Er,Cr:YSGG laser power and surface roughness of lased radicular dentin. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 83–90. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Marcantonio, R.A.C.; Wainwright, M.; Garcia, V.G. LASER in periodontal treatment: Is it an effective treatment or science fiction? Braz. Oral Res. 2021, 35, e099. [Google Scholar] [CrossRef]
- Coluzzi, D.; Convissar, R.; Roshkind, D. Laser Fundamentals. In Principles and Practice of Laser Dentistry, 2nd ed.; Elsevier: St. Louis, MO, USA, 2016; pp. 12–26. [Google Scholar]
- Gutknecht, N.; Van Betteray, C.; Ozturan, S.; Vanweersch, L.; Franzen, R. Laser supported reduction of specific microorganisms in the periodontal pocket with the aid of an Er,Cr:YSGG laser: A pilot study. Sci. World J. 2015, 2015, 450258. [Google Scholar] [CrossRef]
- Ting, C.C.; Fukuda, M.; Watanabe, T.; Sanaoka, A.; Mitani, A.; Noguchi, T. Morphological alterations of periodontal pocket epithelium following Nd:YAG laser irradiation. Photomed. Laser Surg. 2014, 32, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Mulder-van Staden, S.; Holmes, H. In vivo investigation of diode laser application on red complex bacteria in non-surgical periodontal therapy: A split-mouth randomised control trial. Sci. Rep. 2020, 10, 21311. [Google Scholar] [CrossRef] [PubMed]
- Caffesse, R.G.; Sweeney, P.L.; Smith, B.A. Scaling and root planing with and without periodontal flap surgery. J. Clin. Periodontol. 1986, 13, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Sherman, P.; Hutchens, L.; Jewson, L. The Effectiveness of Subgingival Scaling and Root Planing II. Clinical Responses Related to Residual Calculus. J. Periodontol. 1990, 61, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Z.; Li, Y.; Chen, S.S.; Feng, B.; Wang, H.; Wang, Q. Treatment effects and periodontal status of chronic periodontitis after routine Er:YAG laser-assisted therapy. World J. Clin. Cases 2021, 9, 9762–9769. [Google Scholar] [CrossRef]
- Yaneva, B.K.; Zagorchev, P.I.; Firkova, E.I.; Glavinkov, I.T. In Vitro Study of Temperature Changes in Pulp Chamber During Root Planing Procedure Using Er:YAG Laser. Folia Med. 2016, 58, 206–210. [Google Scholar] [CrossRef]
- Drisko, C.L. Periodontal debridement: Still the treatment of choice. J. Evid.-Based Dent. Pract. 2014, 14, 33–41.e1. [Google Scholar] [CrossRef]
- Saito, M.; Narayana, A.S. Signaling reactions induced in human fibroblasts during adhesion to cementum-derived attachment protein. J. Bone Miner. Res. 1999, 14, 65–72. [Google Scholar] [CrossRef]
- Mills, M.P.; Rosen, P.S.; Chambrone, L.; Greenwell, H.; Kao, R.T.; Klokkevold, P.R.; McAllister, B.S.; Reynolds, M.A.; Romanos, G.E.; Wang, H.-L. American Academy of Periodontology best evidence consensus statement on the efficacy of laser therapy used alone or as an adjunct to non-surgical and surgical treatment of periodontitis and peri-implant diseases. J. Periodontol. 2018, 89, 737–742. [Google Scholar] [CrossRef]
- Sasaki, K.M.; Aoki, A.; Masuno, H.; Ichinose, S.; Yamada, S.; Ishikawa, I. Compositional analysis of root cementum and dentin after Er:YAG laser irradiation compared with CO2 lased and intact roots using Fourier transformed infrared spectroscopy. J. Periodontal Res. 2002, 37, 50–59. [Google Scholar] [CrossRef]
- Parker, S.; Cronshaw, M.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Current Concepts of Laser-Oral Tissue Interaction. Dent. J. 2020, 8, 61. [Google Scholar] [CrossRef]
- Schwarz, F.; Sculean, A.; Georg, T.; Reich, E. Periodontal Treatment With an Er:YAG Laser Compared to Scaling and Root Planing. A Controlled Clinical Study. J. Periodontol. 2001, 72, 361–367. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kobayashi, K.; Osada, R.; Sakuraba, E.; Nomura, T.; Arai, T.; Nakamura, J. Effects of Irradiation of an Erbium: YAG Laser on Root Surfaces. J. Periodontol. 1997, 68, 1151–1155. [Google Scholar] [CrossRef]
- Soundarajan, S.; Rajasekar, A. Comparative evaluation of combined efficacy of methylene blue mediated antimicrobial photodynamic therapy (a-PDT) using 660 nm diode laser versus Erbium-chromium-yttrium-scandium-gallium-garnet (Er, Cr:YSGG) laser as an adjunct to scaling and root planing on clinical parameters in supportive periodontal therapy: A randomized split-mouth trial. Photodiagnosis Photodyn. Ther. 2022, 39, 102971. [Google Scholar] [CrossRef]
- Folwaczny, M.; Aggstaller, H.; Mehl, A.; Hickel, R. Removal of bacterial endotoxin from root surface with Er:YAG laser. Am. J. Dent. 2003, 16, 3–5. [Google Scholar]
- Belal, M.H.; Watanabe, H. Comparative study on morphologic changes and cell attachment of periodontitis-affected root surfaces following conditioning with CO2 and Er:YAG laser irradiations. Photomed. Laser Surg. 2014, 32, 553–560. [Google Scholar] [CrossRef]
- Frentzen, M.; Braun, A.; Aniol, D. Er:YAG laser scaling of diseased root surfaces. J. Periodontol. 2002, 73, 524–530. [Google Scholar] [CrossRef]
- Bozbay, E.; Dominici, F.; Gokbuget, A.; Cintan, S.; Guida, L.; Aydin; Mariotti, A.; Pilloni, A. Preservation of root cementum: A comparative evaluation of power-driven versus hand instru ments. Int. J. Dent. Hyg. 2018, 16, 202–209. [Google Scholar] [CrossRef]
- Maruyama, H.; Aoki, A.; Sasaki, K.M.; Takasaki, A.A.; Iwasaki, K.; Ichinose, S.; Oda, S.; Ishikawa, I.; Izumi, Y. The effect of chemical and/or mechanical conditioning on the Er:YAG laser-treated root cementum: Analysis of surface morphology and periodontal ligament fibroblast attachment. Lasers Surg. Med. 2008, 40, 211–222. [Google Scholar] [CrossRef]
- Almehdi, A.; Aoki, A.; Ichinose, S.; Taniguchi, Y.; Sasaki, K.M.; Ejiri, K.; Sawabe, M.; Chui, C.; Katagiri, S.; Izumi, Y. Histological and SEM analysis of root cementum following irradiation with Er:YAG and CO2 lasers. Lasers Med. Sci. 2013, 28, 203–213. [Google Scholar] [CrossRef]

| Group | G1 | G2 | G3 | G4 |
|---|---|---|---|---|
| Energy | 30 mJ | 40 mJ | 50 mJ | 60 mJ |
| Mean | 43.75 A | 50.05 B | 65.56 C | 74.80 D |
| Std. Deviation | 4.89 | 3.72 | 10.35 | 15.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahas, P.; Houeis, S.; Chamboredon, R.; Heysselaer, D.; Zeinoun, T.; Nammour, S. Assessment of the Periodontal Cementum Ablation Depth during Root Planing by an Er:YAG Laser at Different Energy Densities: An Ex Vivo Study. Dent. J. 2023, 11, 116. https://doi.org/10.3390/dj11050116
Nahas P, Houeis S, Chamboredon R, Heysselaer D, Zeinoun T, Nammour S. Assessment of the Periodontal Cementum Ablation Depth during Root Planing by an Er:YAG Laser at Different Energy Densities: An Ex Vivo Study. Dentistry Journal. 2023; 11(5):116. https://doi.org/10.3390/dj11050116
Chicago/Turabian StyleNahas, Paul, Saad Houeis, Remi Chamboredon, Daniel Heysselaer, Toni Zeinoun, and Samir Nammour. 2023. "Assessment of the Periodontal Cementum Ablation Depth during Root Planing by an Er:YAG Laser at Different Energy Densities: An Ex Vivo Study" Dentistry Journal 11, no. 5: 116. https://doi.org/10.3390/dj11050116
APA StyleNahas, P., Houeis, S., Chamboredon, R., Heysselaer, D., Zeinoun, T., & Nammour, S. (2023). Assessment of the Periodontal Cementum Ablation Depth during Root Planing by an Er:YAG Laser at Different Energy Densities: An Ex Vivo Study. Dentistry Journal, 11(5), 116. https://doi.org/10.3390/dj11050116








