In Vitro Evaluation of Tooth-Whitening Potential of Peroxide-Free OTC Dental Bleaching Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection and Preparation
2.2. Staining Procedure
2.3. Bleaching Procedure
2.4. Color Change Measurements and Data Collection
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
- All over-the-counter whitening kits tested in this study, except one, exhibited positive color variation. However, the individual performance differed vastly from one brand to the other, and the overall performance was less effective compared to the conventional carbamide-peroxide-based positive control;
- One product, Hismileteeth, showed a partially negative performance with two specific staining agents. Further research might be needed to understand and investigate the disparity in performance driven by the underlying staining agent;
- The experimental bleaching agent showed the best results of all OCT products tested. These results were close to the positive control with carbamide peroxide.
7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samorodnitzky-Naveh, G.R.; Geiger, S.B.; Levin, L. Patients’ satisfaction with dental esthetics. J. Am. Dent. Assoc. 2007, 138, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, A.M.; Darby, M.L.; McCombs, G.B.; Lynch, C.M. Vital tooth whitening effects on oral health-related quality of life in older adults. J. Dent. Hyg. 2012, 86, 239–247. [Google Scholar] [PubMed]
- Smirnova, M.H. A will to youth: The woman’s anti-aging elixir. Soc. Sci. Med. 2012, 75, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Hattab, F.N.; Qudeimat, M.A.; Al-Rimawi, H.S. Dental discoloration: An overview. J. Esthet. Restor. Dent. 1999, 11, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; Laino, L.; De Stefano, R.; D’Amico, C.; Bocchieri, S.; Amoroso, G.; Isola, G.; Cervino, G. Dental Whitening Gels: Strengths and Weaknesses of an Increasingly Used Method. Gels 2019, 5, 35. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Tatum, S.A. Cosmetic dentistry. Curr. Opin. Otolaryngol. Head Neck Surg. 2006, 14, 254–259. [Google Scholar] [CrossRef]
- Demarco, F.F.; Meireles, S.S.; Masotti, A.S. Over-the-counter whitening agents: A concise review. Braz. Oral Res. 2009, 23, 64–70. [Google Scholar] [CrossRef]
- Greenwall, V.V.; Fredman, L.; Gordan, G. Bleaching Techniques in Restorative Dentistry: An Illustrated Guide; Martin Dunitz: London, UK, 2001.
- Kugel, G. Over-the-counter tooth-whitening systems. Compend. Contin. Educ. Dent. 2003, 24, 376–382. [Google Scholar]
- Pintado-Palomino, K.; Filho, O.P.; Zanotto, E.D.; Tirapelli, C. A clinical, randomized, controlled study on the use of desensitizing agents during tooth bleaching. J. Dent. 2015, 43, 1099–1105. [Google Scholar] [CrossRef]
- The Council of the European Union. Council Directive 2011/84/EU of 20 September 2011. Off. J. Eur. Union 2011, 7, 36–38. [Google Scholar]
- Dietschi, D.; Rossier, S.; Krejci, I. In vitro colorimetric evaluation of the efficacy of various bleaching methods and products. J. Prosthet. Dent. 2007, 97, 317. [Google Scholar] [CrossRef]
- Gregor, L.; Krejci, I.; Di Bella, E.; Feilzer, A.J.; Ardu, S. Silorane, ormocer, methacrylate and compomer long-term staining susceptibility using ΔE and ΔE 00 colour-difference formulas. Odontology 2016, 104, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Ardu, S.; Braut, V.; Gutemberg, D.; Krejci, I.; Dietschi, D.; Feilzer, A.J. A long-term laboratory test on staining susceptibility of esthetic composite resin materials. Quintessence Int. 2010, 41, 59. [Google Scholar]
- Ardu, S.; Braut, V.; Di Bella, E.; Lefever, D. Influence of background on natural tooth colour coordinates: An in vivo evaluation. Odontology 2014, 102, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Ardu, S.; Duc, O.; Di Bella, E.; Krejci, I. Color stability of recent composite resins. Odontology 2017, 105, 29–35. [Google Scholar] [CrossRef]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Della Bona, A.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Perez, M.D.M. Color Difference Thresholds in Dentistry. J. Esthet. Restor. Dent. 2015, 27, S1–S9. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Um, C.M.; Ruyter, I.E. Staining of resin-based veneering materials with coffee and tea. Quintessence Int. 1991, 22, 377–386. [Google Scholar]
- Fujita, M.; Kawakami, S.; Noda, M.; Sano, H. Color change of newly developed esthetic restorative material immersed in food-simulating solutions. Dent. Mater. J. 2006, 25, 352–359. [Google Scholar] [CrossRef]
- Greenwall-Cohen, J.; Francois, P.; Silikas, N.; Greenwall, L.; Le Goff, S.; Attal, J.-P. The safety and efficacy of “over the counter” bleaching products in the UK. Br. Dent. J. 2019, 226, 271–276. [Google Scholar] [CrossRef]
- Denmark, S.E.; Forbes, D.C.; Hays, D.S.; DePue, J.S.; Wilde, R.G. Catalytic Epoxidation of Alkenes with Oxone. J. Org. Chem. 1995, 60, 1391–1407. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9860421, Phthalimidoperoxycaproic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phthalimidoperoxycaproic-acid (accessed on 30 October 2020).
- Sorrenti, V.; Randazzo, C.L.; Caggia, C.; Ballistreri, G.; Romeo, F.V.; Fabroni, S.; Timpanaro, N.; Raffaele, M.; Vanella, L. Beneficial Effects of Pomegranate Peel Extract and Probiotics on Pre-adipocyte Differentiation. Front. Microbiol. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Kusculuo, N.G.; Benli, H. Staining Effect of Pomegranate Flower Extract on Human Blood Cells: First Results. J. Environ. Sci. Eng. A 2017, 6, 249–251. [Google Scholar]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Toman, J.; Bazin, I.; Roubal, T. Transfer of ochratoxin A into tea and coffee beverages. Toxins 2014, 6, 3438–3453. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Rahul Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Lussi, A.; Jaeggi, T.; Jaeggi-Scharer, S. Prediction of the erosive potential of some beverages. Caries Res. 1995, 29, 349–354. [Google Scholar] [CrossRef]
- Ablal, M.A.; Adeyemi, A.A.; Jarad, F.D. The whitening effect of chlorine dioxide—An in vitro study. J. Dent. 2013, 41, e76–e81. [Google Scholar] [CrossRef]
- Jurema, A.L.B.; Claudino, E.S.; Torres, C.R.G.; Bresciani, E.; Caneppele, T.M.F. Effect of over-the-counter whitening products associated or not with 10% carbamide peroxide on color change and microhardness: In vitro study. J. Contemp. Dent. Pract. 2018, 19, 359–366. [Google Scholar]
- Mosquim, V.; Souza, B.M.; Foratori, G.A.; Wang, L.; Magalhães, A.C. The abrasive effect of commercial whitening toothpastes on eroded enamel. Am. J. Dent. 2017, 30, 142–146. [Google Scholar]
- Guinesi, A.S.; Andolfatto, C.; Idomeo, B.F.; Arnaldo, A.C.; Juliano, P.F.; Roberta, V.F. Ozonized Oils: A qualitative and quantitative analysis. Braz. Dent. J. 2011, 22, 37–40. [Google Scholar] [CrossRef]
- AL-Omiri, M.K.; Lamfon, H.A.; Al Nazeh, A.A.; Kielbassa, A.M.; Lynch, E. Randomized clinical trial on the comparison of bleaching outcomes using either ozone or hydrogen peroxide. Quintessence Int. 2018, 49, 625–634. [Google Scholar]
- Suh, Y.; Patel, S.; Kaitlyn, R.; Gandhi, J.; Joshi, G.; Smith, N.L.; Khan, S.A. Clinical utility of ozone therapy in dental and oral medicine. Med. Gas Res. 2019, 9, 163–167. [Google Scholar] [PubMed]
| Group | Staining Agent | Manufacturer | Batch Number | Proportion |
|---|---|---|---|---|
| Group A | Coffee | Ristretto, Nespresso, Nestlé, Switzerland | 0272378606 | 60 mL |
| Group B | Tea | Twining Earl Gray tea, London, England | 0000579251 | 3 tea bags in 60 mL of water |
| Group C | Red Wine | Côte du Rhône (DOC), Les arènes, Vacqueyras | 1306471D | 60 mL |
| Group D | Curry | Curry Bio Natura plan Coop | 1291177 | 5 g curry in 60 mL water |
| Group E | Distilled Water | N/A | N/A | 60 mL |
| Group | Product | Manufacturer | Ingredients | Active Agent |
|---|---|---|---|---|
| N°1 | MeaWhite kit teeth whitening | Plastimea SA (Brussel, Belgium) | Glycerin, Propylene glycol, Purified water, Hazel extract, Sodium phytate, Citric acid, carboxymethyl | Citric Acid |
| N°2 | iWhite instant teeth whitening | Sylphar NV (Deurle, Belgium) | Aqua, Hydrated Silica, Glycerin, Sorbitol, Chondrus Crispus Powder, PEG-40 Hydrogenated Castor Oil, Aroma, Phthalimidoperoxycaproic Acid, Citric Acid, Methylparaben, Acrylates/Acrylamide Copolymer, Paraffinum Liquidum, Xylitol, Calcium Lactate, Calcium Gluconate, Potassium Acesulfame, Polysorbate 85, BHT | Phthalimidoperoxycaproic Acid, Citric Acid |
| N°3 | PAP pure | Cosmolab (Zurich, Switzerland) | Glycerin, propylene glycol, maltodextrin, phthalimidoperoxycaproic acid, acrylates/C10–30 alkyl acrylate cross polymer, menthe arvensps leaf oil, mica, CI 77891, menthe piperita oil sodium saccharin | Phthalimidoperoxycaproic Acid (10–15%) |
| N°4 | Opalescence PF 16% regular | Ultradent (Dardilly, France) | Carbamide peroxide 16%, Glycerin, Water, Urea, Xylitol, Carbomer, PEG-6, Sodium Hydroxide, EDTA, Potassium Nitrate, Sodium Fluoride | Carbamide Peroxyde 16% |
| N°5 | EXP1 | CUMD (Geneva, Switzerland) | 0.1% H2O2, Doping agent | 0.1% H2O2 |
| N°6 | HiSmile teeth whitening kit | HiSmile Pty Ltd (Goldcoast, Australia) | Sorbitol, Water, Phthalimidoperoxycaproic Acid, Propylene Glycol, Glycerin, Potassium Nitrate, Polyethylene Glycol-8, Hydroxyapatite, Sodium Carboxymethyl Cellulose, Hydroxyethyl Cellulose, Xanthan Gum, Peppermint Essence, Saccharin Sodium, Methylparaben, Sodium Bicarbonate, Aloe Leaf Extract, Chamomile Extract, Pomegranate Seed Extract, Propylparaben | Phthalimidoperoxycaproic Acid |
| N°7 | Lubricating Gel | K-Y Johnson & Johnson | Water, Glycerine, Propylene Glycol, Hydroxyethylcellulose, Methylparaben, Sodium phosphate, Disodium phosphate, Propylparaben, Tetrasodium EDTA | N/A |
| N°8 | oZoral Gel oral | Innovares Srl (Sant’Ilario d’Enza, Italy) | Water, Ozonized Sunflower Seed Oil, Aroma, Glycerin, Carbomer, Polycarbophil, Sodium Hydroxide, Sodium Saccharin, Glyceryl Caprylate, Tocopherol, Ascorbyl Palmitate, Disodium EDTA, Limonene, Linalool | Ozonized Sunflower Seed Oil |
| Group | Product | Code | Batch Number | Instruction for Use | Experimental Application | Light Activation |
|---|---|---|---|---|---|---|
| N°1 | MeaWhite kit teeth whitening | MEA | 93/42/EEC2007/47/EC | 20 × 20 min | 20 × 20 min | Yes |
| N°2 | iWhite instant teeth whitening | IWH | AAA15605-2020 | 5 × 20 min | 10 × 20 min | No |
| N°3 | PAP pure | PAP pure | No batch number as is has been freshly produced in the manufacturer’s laboratory | 10 × 20 min | 10 × 20 min | Yes |
| N°4 | Opalescence PF 16% regular | OPL | BGX34 | 7 × 5 h | 10 × 20 min | No |
| N°5 | EXP1 | EXP1 | No batch number as is has been freshly produced in the CUMD laboratory | 10 × 20 min | 10 × 20 min | No |
| N°6 | HiSmile teeth whitening kit | HST | 111042019 | 6 × 10 min | 20 × 10 min | Yes |
| N°7 | Gylcerin | GLY | 8351914 | N/A | 10 × 20 min | No |
| N°8 | oZoral Gel oral | OZG | 30318 | 7 × 20 min | 10 × 20 min | No |
| White BG | Distilled Water | Coffee | Curry + Oil | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Description | t1-t2 | t1-t3 | t1-t4 | t1-t2 | t1-t3 | t1-t4 | t1-t2 | t1-t3 | t1-t4 |
| G_1 | MeaWhite | 1.67 A (1.05) | 2.44 A (1.87) | 2.97 A (2.00) | 1.84 B (0.92) | 1.97 A (1.89) | 2.89 A (3.18) | 2.74 A (1.55) | 2.64 A (1.12) | 2.66 A (1.31) |
| G_2 | Iwhite | 1.21 A (0.79) | 1.97 A (1.9) | 2.88 A (3.33) | 3.26 B (2.42) | 3.18 A (2.2) | 3.56 A (2.61) | 2.81 A (0.93) | 3.34 A (1.22) | 3.4 A (0.69) |
| G_3 | PAP pure | 1.4 A (0.50) | 1.34 A (0.61) | 1.78 A (0.58) | 2.52 B (1.55) | 2.34 A (1.2) | 2.13 A (1.14) | 2.33 A (0.8) | 2.24 A (0.62) | 2.13 A (0.6) |
| G_4 | Opalescence PF | 2.56 A (1.11) | 2.55 A (1.59) | 3.26 A (1.53) | 2.47 B (1.28) | 2.15 A (1.32) | 3.51 A (1.95) | 2.93 A (0.75) | 2.63 A (0.95) | 3.45 A (1.02) |
| G_5 | EXP1 | 1.36 A (0.73) | 2.01 A (1.12) | 3.04 A (0.88) | 4.24 A (1.64) | 3.02 A (1.07) | 3.69 A (1.68) | 3.3 A (1.66) | 3.41 A (0.89) | 5.07 A/B (1.71) |
| G_6 | Hismileteeth | 1.32 A (0.7) | 1.35 A (0.75) | 1.90 A (0.55) | 1.64 B (0.95) | 2.1 A (0.82) | 2.13 A (0.94) | 2.76 A (1.7) | 3.00 A (1.03) | 3.67 A (1.73) |
| G_7 | Placebo | 1.74 A (1.05) | 1.42 A (0.51) | 1.56 A (0.64) | 3.54 B (5.87) | 1.69 A (0.86) | 1.54 A (0.78) | 2.07 A (0.85) | 2.63 A (0.83) | 2.53 A (1.01) |
| G_8 | oZoral gel | 1.93 A (1.07) | 1.42 A (0.77) | 1.04 A (0.7) | 2.89 B (2.38) | 2.83 A (2.45) | 3.05 A (2.23) | 3.05 A (1.05) | 3.53 A (1.45) | 3.62 A (1.04) |
| White BG | Red Wine | Tea | Overall | |||||||
| Group | Description | t1-t2 | t1-t3 | t1-t4 | t1-t2 | t1-t3 | t1-t4 | t1-t2 | t1-t3 | t1-t4 |
| G_1 | MeaWhite | 5.43 B (6.79) | 4.67 B (2.29) | 5.49 C (3.15) | 3.7 B (3.51) | 2.89 C (2.77) | 2.59 C (2.82) | 3.08 B (3.69) | 2.92 C (2.2) | 3.32 C (2.74) |
| G_2 | Iwhite | 5.27 B (2.63) | 5.02 B (3.02) | 6.45 B (2.35) | 3.24 B (4.08) | 2.14 C (1.08) | 2.42 C (1.6) | 3.16 B (2.73) | 3.13 B (2.23) | 3.74 C (2.64) |
| G_3 | PAP pure | 3.83 C (2.34) | 4.57 B (2.59) | 5.12 C (2.56) | 2.08 C (0.8) | 1.94 C (0.97) | 2.21 C (0.9) | 2.43 B (1.55) | 2.49 C (1.75) | 2.67 D (1.81) |
| G_4 | Opalescence PF | 7.81 A (3.77) | 9.43 A (4.6) | 11.2 A (4.39) | 8.37 A (6.69) | 8.54 A (7.08) | 10.17 A (6.51) | 4.83 A (4.35) | 5.06 A (4.98) | 6.32 A (5.09) |
| G_5 | EXP1 | 4.07 C (2.26) | 3.68 B (2.46) | 5.86 C (2.24) | 3.02 B (1.41) | 2.89 C (0.97) | 4.27 B (1.31) | 3.2 B (1.87) | 3.00 B (1.49) | 4.38 B (1.86) |
| G_6 | Hismileteeth | 6.44 A (2.97) | 7.58 A (2.67) | 7.7 B (2.45) | 7.99 A (8.27) | 5.28 B (4.61) | 5.31 B (3.69) | 4.03 A (4.75) | 3.86 B (3.32) | 4.14 B (3.03) |
| G_7 | Placebo | 3.53 C (1.99) | 3.75 B (3.03) | 2.97 D (1.94) | 1.82 C (0.74) | 1.91 C (1.13) | 2.09 C (1.08) | 2.54 B (2.88) | 2.28 C (1.73) | 2.14 D (1.27) |
| G_8 | oZoral gel | 4.28 C (3.44) | 2.89 B (1.27) | 2.86 D (1.63) | 1.48 C (1.45) | 1.19 C (1.63) | 2.56 C (2.31) | 2.73 B (2.25) | 2.37 C (1.8) | 2.62 D (1.86) |
| Black BG | Distilled Water | Coffee | Curry + Oil | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Description | t1-t2 | t1-t3 | t1-t4 | t1-t2 | t1-t3 | t1-t4 | t1-t2 | t1-t3 | t1-t4 |
| G_1 | MeaWhite | 2.93 A (2.33) | 2.87 A (2.57) | 3.11 A (2.34) | 1.72 A (1.19) | 1.38 B (1.05) | 3.71 A (4.26) | 3.84 A (2.4) | 3.25 A (1.55) | 3.8 B (1.91) |
| G_2 | Iwhite | 2.31 A (2.29) | 2.97 A (2.55) | 3.56 A (3.26) | 3.13 A (1.25) | 4.59 A (1.83) | 4 A (2.81) | 2.92 A (1.69) | 3.66 A (1.8) | 3.67 B (1.05) |
| G_3 | PAP pure | 2.33 A (1.91) | 2.79 A (2.12) | 2.3 A (1.94) | 2.51 A (1.62) | 2.29 B (1.76) | 2.5 A (1.61) | 2.27 A (1.14) | 2.4 A (1.4) | 2.36 B (1.18) |
| G_4 | Opalescence PF | 4.47 A (4.27) | 4.27 A (4.44) | 4.83 A (4.41) | 3.84 A (3.37) | 3.16 A/B (2.11) | 3.4 A (1.61) | 3.1 A (1.64) | 3.16 A (1.59) | 3.57 B (1.79) |
| G_5 | EXP1 | 2.03 A (1.09) | 2.62 A (1.89) | 3.65 A (1.24) | 3.06 A (1.21) | 2.25 B (0.94) | 3.05 A (1.64) | 3.89 A (1.58) | 3.27 A (0.85) | 6.02 A (1.91) |
| G_6 | Hismileteeth | 1.52 A (1.01) | 1.29 A (0.59) | 2.08 A (1.29) | 1.93 A (1.47) | 2.17 B (1.29) | 2.11 A (1.37) | 2.81 A (1.34) | 3.19 A (1.00) | 4.17 B (1.27) |
| G_7 | Placebo | 2.88 A (1.63) | 1.79 A (1.57) | 1.91 A (1.71) | 2.71 A (1.81) | 1.4 B (0.88) | 1.73 A (1.05) | 2.85 A (1.27) | 3.19 A (1.39) | 3.13 B (1.49) |
| G_8 | oZoral gel | 2.14 A (1.11) | 1.18 A (0.83) | 1.25 A (0.94) | 2.00 A (1.5) | 2.4 B (1.44) | 2.43 A (1.08) | 4.69 A (1.74) | 5.04 A (2.39) | 5.86 A (1.68) |
| Black BG | Red Wine | Tea | Overall | |||||||
| Group | Description | t1-t2 | t1-t3 | t1-t4 | t1-t2 | t1-t3 | t1-t4 | t1-t2 | t1-t3 | t1-t4 |
| G_1 | MeaWhite | 4.77 A (2.45) | 5.55 B (2.73) | 6.28 B (2.80) | 3.92 C (3.52) | 4.53 C (3.46) | 4.37 B (3.26) | 3.44 B (2.62) | 3.51 B (2.75) | 4.25 B (3.12) |
| G_2 | Iwhite | 4.34 A (2.04) | 5.19 B (2.51) | 5.83 B (3.10) | 3.77 C (2.92) | 3.77 C (1.84) | 4.06 B (2.13) | 3.29 B (2.16) | 4.04 B (2.20) | 4.23 B (2.64) |
| G_3 | PAP pure | 6.27 A (3.22) | 6.09 B (3.59) | 5.92 B (4.02) | 2.71 C (2.73) | 2.81 C (2.86) | 2.98 C (2.86) | 3.22 B (2.67) | 3.28 B (2.79) | 3.21 C (2.81) |
| G_4 | Opalescence PF | 6.83 A (2.57) | 8.16 A (2.94) | 9.39 A (2.59) | 6.05 B (4.11) | 6.14 B (3.6) | 7.73 A (5.1) | 4.86 A (3.51) | 4.98 A (3.57) | 5.78 A (4.07) |
| G_5 | EXP1 | 4.78 A (2.17) | 3.77 C (2.56) | 5.28 B (2.44) | 2.72 C (1.64) | 3.10 C (1.16) | 4.47 B (1.46) | 3.30 B (1.81) | 3.00 B/C (1.65) | 4.49 B (2.04) |
| G_6 | Hismileteeth | 5.40 A (2.64) | 6.34 B (2.52) | 5.43 B (2.77) | 9.97 A (10.33) | 9.17 A (9.82) | 7.50 A (6.77) | 4.33 A (5.66) | 4.43 A (5.33) | 4.26 B (3.91) |
| G_7 | Placebo | 6.37 A (3.45) | 3.13 C (1.80) | 3.03 C (1.63) | 1.59 D (0.76) | 1.62 D (0.99) | 1.77 C (1.2) | 3.28 B (2.53) | 2.23 C (1.53) | 2.31 C (1.53) |
| G_8 | oZoral gel | 2.64 B (1.34) | 2.65 C (0.92) | 3.68 C (1.51) | 1.43 D (0.82) | 2.02 D (1.58) | 2.58 C (2.53) | 2.58 B (1.72) | 2.66 C (1.98) | 3.16 C (2.23) |
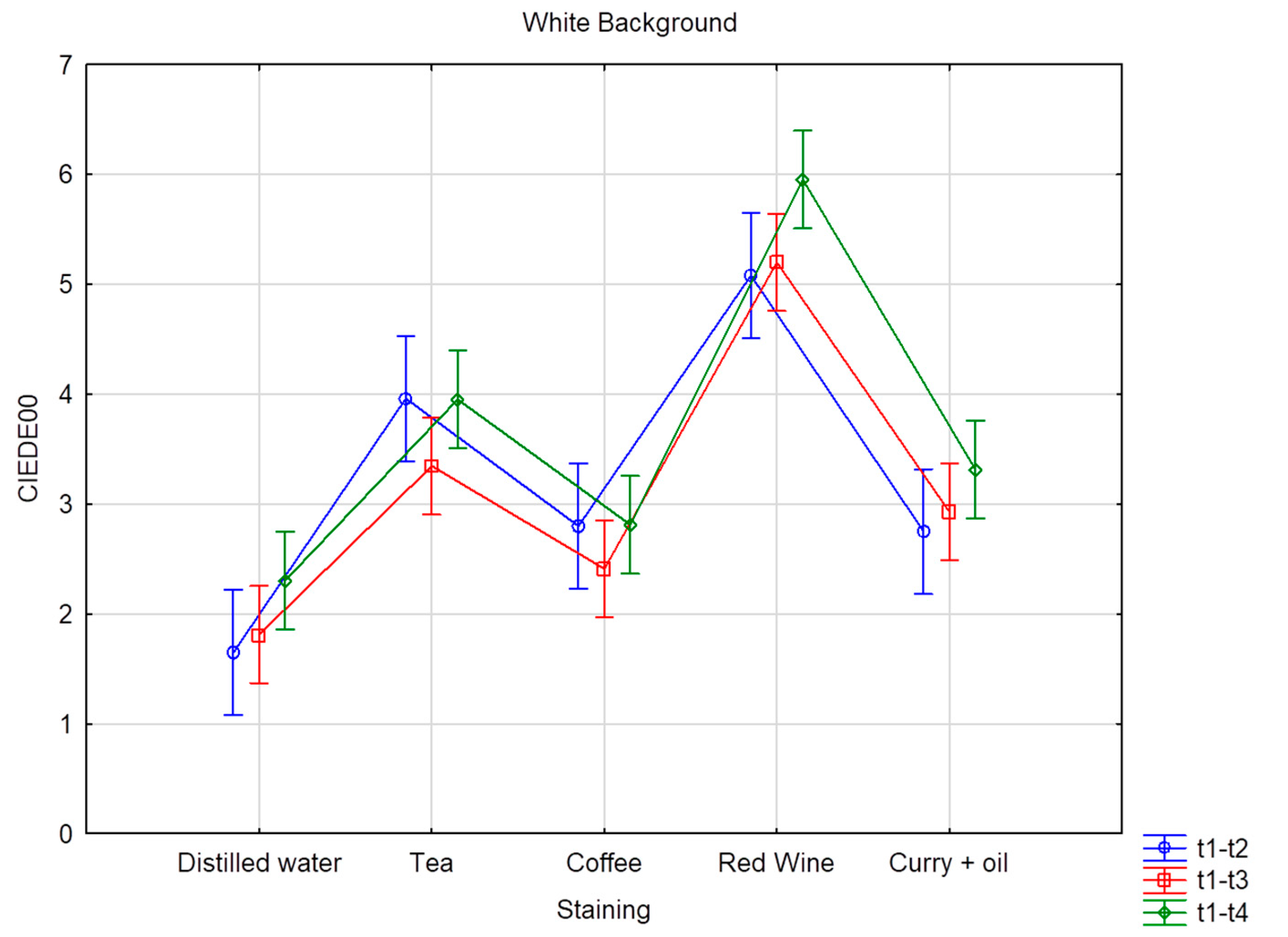
| White Background | ||||||
|---|---|---|---|---|---|---|
| Mean t1-t2 | SD t1-t2 | Mean t1-t3 | SD t1-t3 | Mean t1-t4 | SD t1-t4 | |
| Distilled water | 1.65 D | 0.96 | 1.81 D | 1.30 | 2.30 C | 1.70 |
| Coffee | 2.80 C | 2.64 | 2.41 C | 1.61 | 2.81 C | 2.05 |
| Curry + oil | 2.75 C | 1.23 | 2.93 C | 1.09 | 3.31 B | 1.44 |
| Red Wine | 5.08 A | 3.71 | 5.20 A | 3.46 | 5.96 A | 3.63 |
| Tea | 3.96 B | 4.85 | 3.35 B | 3.89 | 3.95 B | 3.93 |
| Total value | 3.25 | 3.26 | 3.14 | 2.79 | 3.67 | 3.02 |
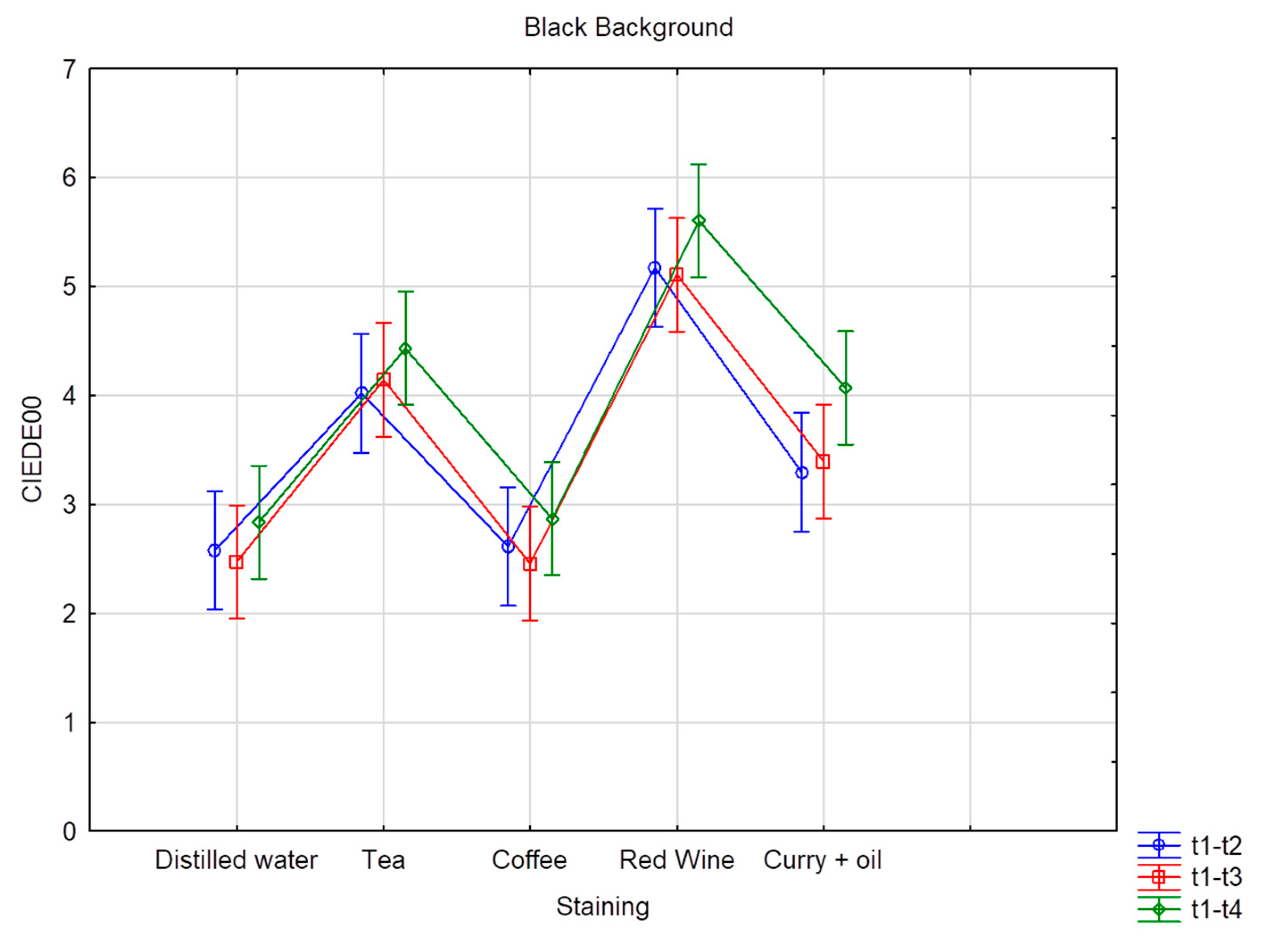
| Black Background | ||||||
|---|---|---|---|---|---|---|
| Mean t1-t2 | SD t1-t2 | Mean t1-t3 | SD t1-t3 | Mean t1-t4 | SD t1-t4 | |
| Distilled water | 2.58 C | 2.27 | 2.47 C | 2.46 | 2.84 C | 2.56 |
| Coffee | 2.61 C | 1.86 | 2.46 C | 1.72 | 2.87 C | 2.23 |
| Curry + oil | 3.30 C | 1.74 | 3.40 B | 1.66 | 4.07 B | 1.92 |
| Red Wine | 5.17 A | 2.78 | 5.11 A | 3.01 | 5.61 A | 3.16 |
| Tea | 4.02 B | 5.01 | 4.14 B | 4.63 | 4.43 B | 4.04 |
| Total value | 3.54 | 3.13 | 3.52 | 3.07 | 3.96 | 3.05 |
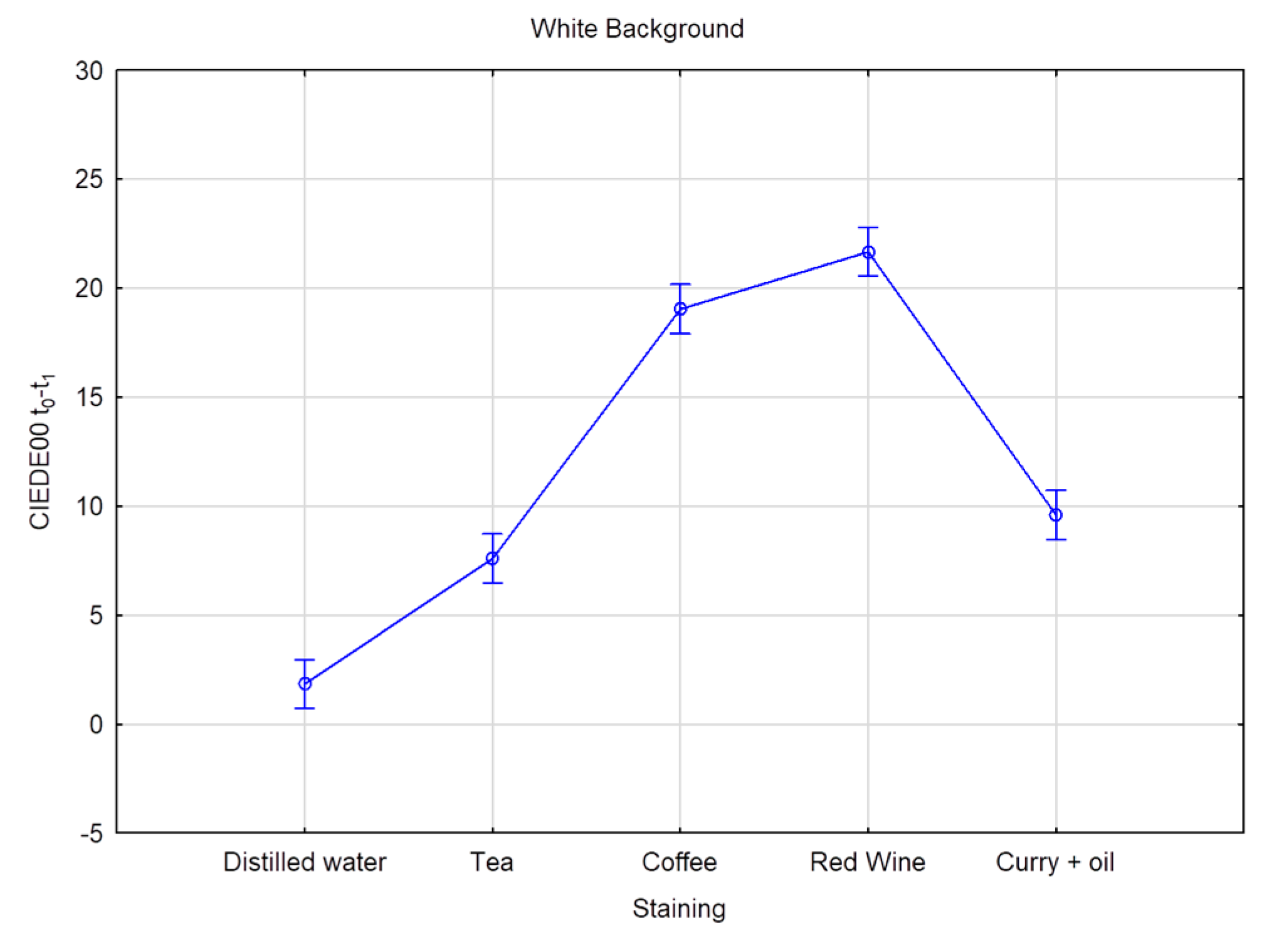
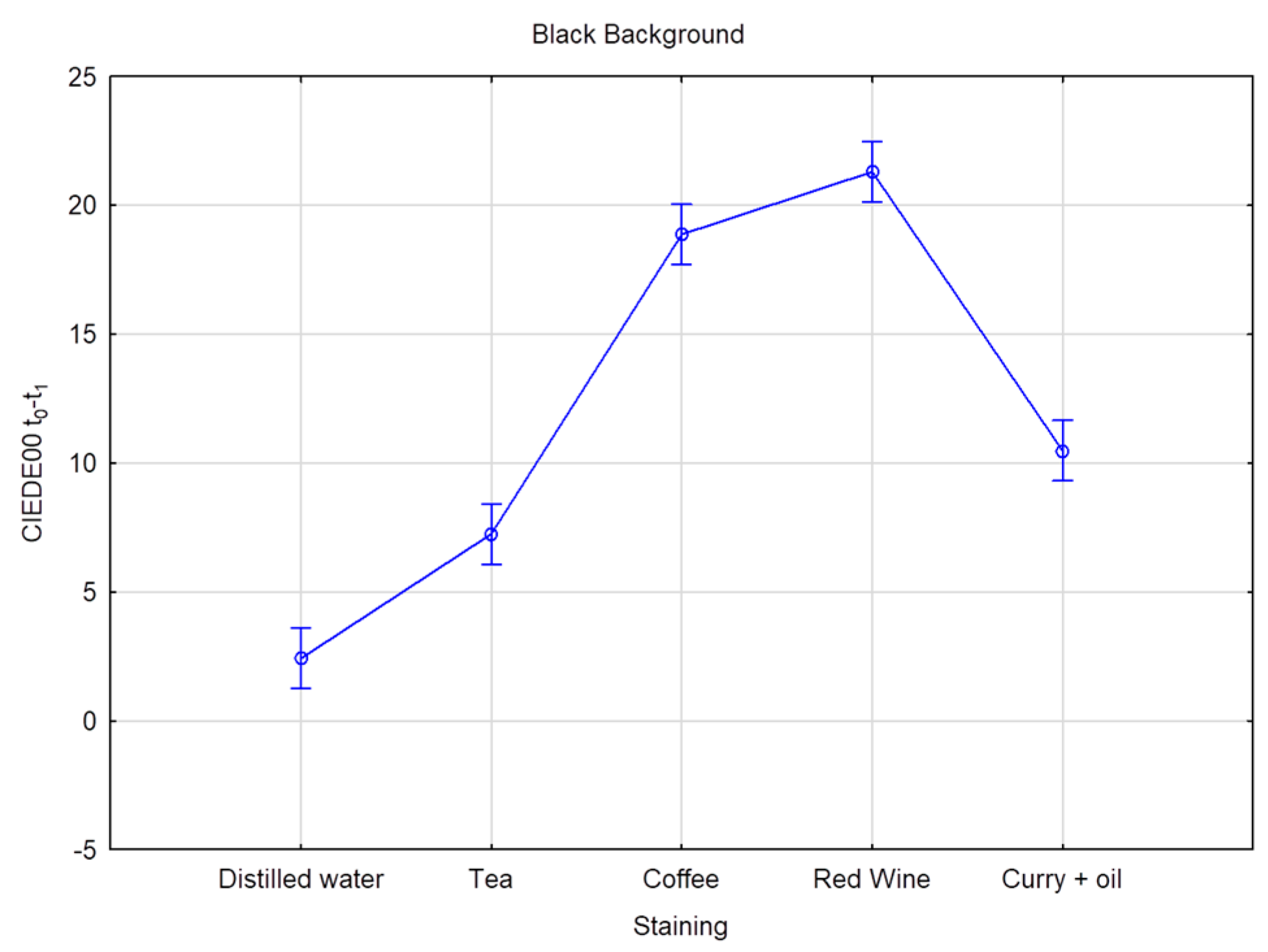
| White Background | Black Background | |||
|---|---|---|---|---|
| Mean t0-t1 | SD | Mean t0-t1 | SD | |
| Distilled water | 1.85 E | 1.34 | 2.42 E | 2.64 |
| Coffee | 19.05 B | 8.74 | 18.88 B | 9.09 |
| Curry + oil | 9.60 C | 3.44 | 10.48 C | 4.32 |
| Red Wine | 21.67 A | 6.39 | 21.30 A | 6.45 |
| Tea | 7.60 D | 5.24 | 7.25 D | 4.42 |
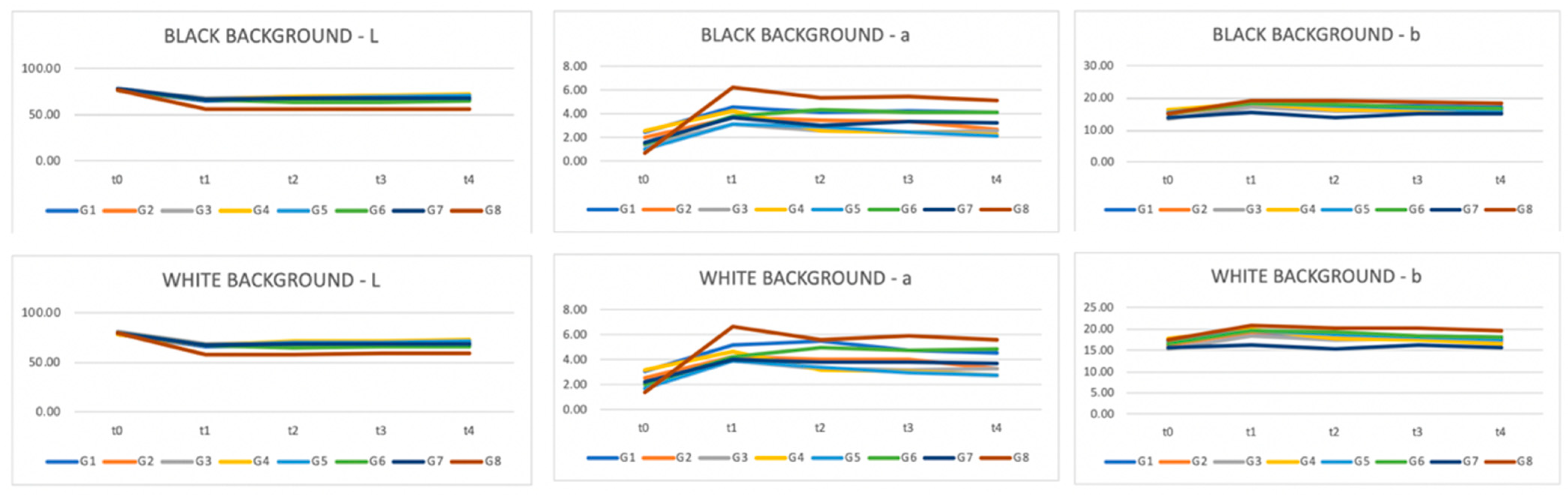
| L | BLACK | |||||
| t0 | t1 | t2 | t3 | t4 | ||
| G1 | 77.36 | 64.51 | 66.05 | 66.67 | 67.25 | |
| G2 | 77.70 | 66.68 | 69.29 | 70.56 | 70.87 | |
| G3 | 77.93 | 67.44 | 69.05 | 68.97 | 69.02 | |
| G4 | 76.85 | 66.16 | 70.16 | 70.45 | 71.85 | |
| G5 | 77.96 | 67.09 | 68.18 | 68.85 | 70.55 | |
| G6 | 77.55 | 66.47 | 64.02 | 63.90 | 64.96 | |
| G7 | 77.79 | 66.73 | 67.91 | 67.72 | 67.65 | |
| G8 | 76.97 | 55.76 | 56.10 | 56.41 | 57.05 | |
| WHITE | ||||||
| t0 | t1 | t2 | t3 | t4 | ||
| G1 | 78.42 | 65.81 | 67.04 | 67.55 | 68.01 | |
| G2 | 79.14 | 68.19 | 70.52 | 71.06 | 71.78 | |
| G3 | 80.58 | 69.08 | 70.47 | 70.81 | 70.41 | |
| G4 | 78.14 | 67.12 | 71.12 | 71.55 | 73.22 | |
| G5 | 79.47 | 68.07 | 69.69 | 69.99 | 71.62 | |
| G6 | 79.18 | 67.80 | 65.45 | 65.63 | 65.84 | |
| G7 | 79.36 | 67.88 | 68.36 | 68.60 | 69.19 | |
| G8 | 80.01 | 58.31 | 58.16 | 58.93 | 58.96 | |
| a | BLACK | |||||
| t0 | t1 | t2 | t3 | t4 | ||
| G1 | 2.49 | 4.57 | 4.18 | 4.24 | 4.17 | |
| G2 | 2.06 | 3.71 | 3.49 | 3.33 | 2.68 | |
| G3 | 1.36 | 3.11 | 2.55 | 2.51 | 2.64 | |
| G4 | 2.54 | 4.20 | 2.64 | 2.49 | 2.25 | |
| G5 | 1.07 | 3.20 | 2.96 | 2.53 | 2.20 | |
| G6 | 1.46 | 3.85 | 4.34 | 4.18 | 4.14 | |
| G7 | 1.58 | 3.76 | 2.99 | 3.33 | 3.27 | |
| G8 | 0.65 | 6.29 | 5.40 | 5.46 | 5.17 | |
| WHITE | ||||||
| t0 | t1 | t2 | t3 | t4 | ||
| G1 | 3.10 | 5.19 | 5.55 | 4.73 | 4.53 | |
| G2 | 2.55 | 4.22 | 4.08 | 4.02 | 3.33 | |
| G3 | 2.01 | 3.95 | 3.34 | 3.23 | 3.34 | |
| G4 | 3.15 | 4.63 | 3.21 | 3.12 | 2.76 | |
| G5 | 1.70 | 3.92 | 3.44 | 3.00 | 2.76 | |
| G6 | 2.03 | 4.27 | 4.94 | 4.73 | 4.84 | |
| G7 | 2.21 | 4.08 | 3.78 | 3.83 | 3.67 | |
| G8 | 1.41 | 6.67 | 5.65 | 5.92 | 5.61 | |
| b | BLACK | |||||
| t0 | t1 | t2 | t3 | t4 | ||
| G1 | 16.21 | 17.02 | 17.49 | 17.50 | 17.07 | |
| G2 | 14.99 | 18.14 | 17.44 | 16.88 | 15.96 | |
| G3 | 13.50 | 17.00 | 15.74 | 15.94 | 16.30 | |
| G4 | 16.29 | 18.54 | 16.38 | 16.46 | 15.71 | |
| G5 | 15.56 | 18.20 | 17.54 | 16.81 | 16.13 | |
| G6 | 15.20 | 18.47 | 17.91 | 17.28 | 16.59 | |
| G7 | 14.04 | 15.71 | 14.06 | 15.31 | 14.99 | |
| G8 | 15.02 | 19.19 | 19.15 | 18.88 | 18.39 | |
| WHITE | ||||||
| t0 | t1 | t2 | t3 | t4 | ||
| G1 | 17.37 | 18.65 | 18.31 | 18.47 | 18.15 | |
| G2 | 16.33 | 19.42 | 18.96 | 18.44 | 17.23 | |
| G3 | 15.24 | 18.47 | 17.37 | 17.67 | 17.90 | |
| G4 | 17.69 | 19.91 | 17.87 | 17.58 | 16.60 | |
| G5 | 17.10 | 19.62 | 18.68 | 17.94 | 17.33 | |
| G6 | 16.63 | 19.67 | 19.24 | 18.51 | 18.09 | |
| G7 | 15.58 | 16.23 | 15.49 | 16.14 | 15.79 | |
| G8 | 17.39 | 20.90 | 20.07 | 20.16 | 19.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grillon, M.; Di Bella, E.; Krejci, I.; Ardu, S. In Vitro Evaluation of Tooth-Whitening Potential of Peroxide-Free OTC Dental Bleaching Agents. Dent. J. 2023, 11, 89. https://doi.org/10.3390/dj11040089
Grillon M, Di Bella E, Krejci I, Ardu S. In Vitro Evaluation of Tooth-Whitening Potential of Peroxide-Free OTC Dental Bleaching Agents. Dentistry Journal. 2023; 11(4):89. https://doi.org/10.3390/dj11040089
Chicago/Turabian StyleGrillon, Marlene, Enrico Di Bella, Ivo Krejci, and Stefano Ardu. 2023. "In Vitro Evaluation of Tooth-Whitening Potential of Peroxide-Free OTC Dental Bleaching Agents" Dentistry Journal 11, no. 4: 89. https://doi.org/10.3390/dj11040089
APA StyleGrillon, M., Di Bella, E., Krejci, I., & Ardu, S. (2023). In Vitro Evaluation of Tooth-Whitening Potential of Peroxide-Free OTC Dental Bleaching Agents. Dentistry Journal, 11(4), 89. https://doi.org/10.3390/dj11040089









