Efficacy/Safety of the Use of Glucocorticoids in Oral and Maxillofacial Surgery
Abstract
1. Introduction
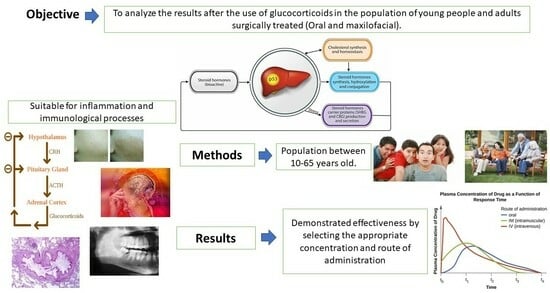
1.1. General Objective
1.2. Specific Objectives
- Identify the applications of glucocorticoids in oral and maxillofacial surgery in adolescents and adults.
- Determine the appropriate dose to be administered in oral and maxillofacial surgery in adolescent and adult patients where there is no presence of other previous pathologies.
- Examine the advantages and disadvantages of the different pharmaceutical forms of glucocorticoids.
- Highlight the interactions of glucocorticoids with other drugs.
- Describe the effectiveness when glucocorticoids are administered to attend oral manifestations and maxillofacial diseases in adults (aged 16–65 years).
- Study the adverse reactions of glucocorticoids after prolonged use in patients undergoing oral and maxillofacial surgery.
2. Methodology
2.1. Review Type
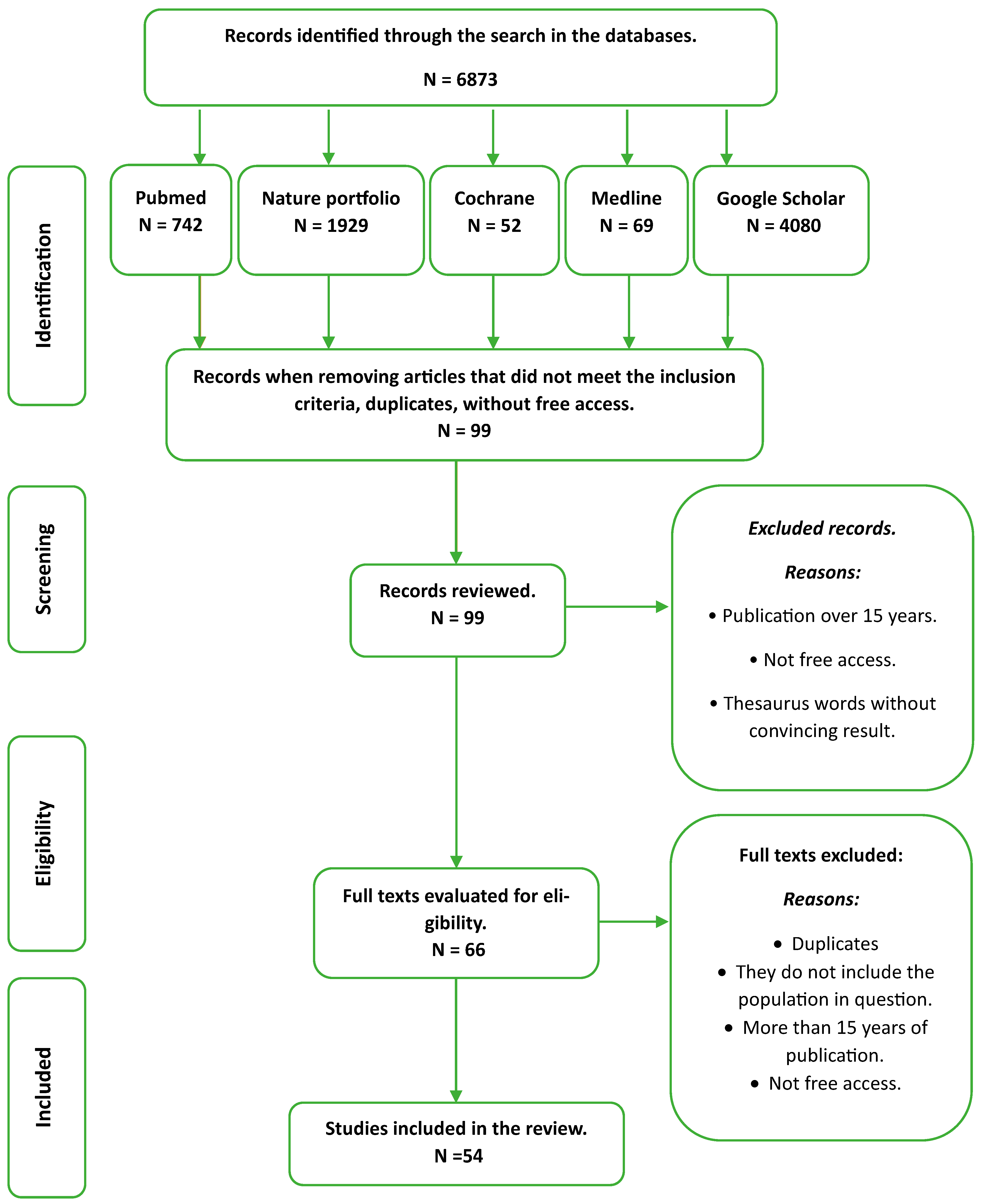
2.2. Search Strategy
2.3. Selection Criteria
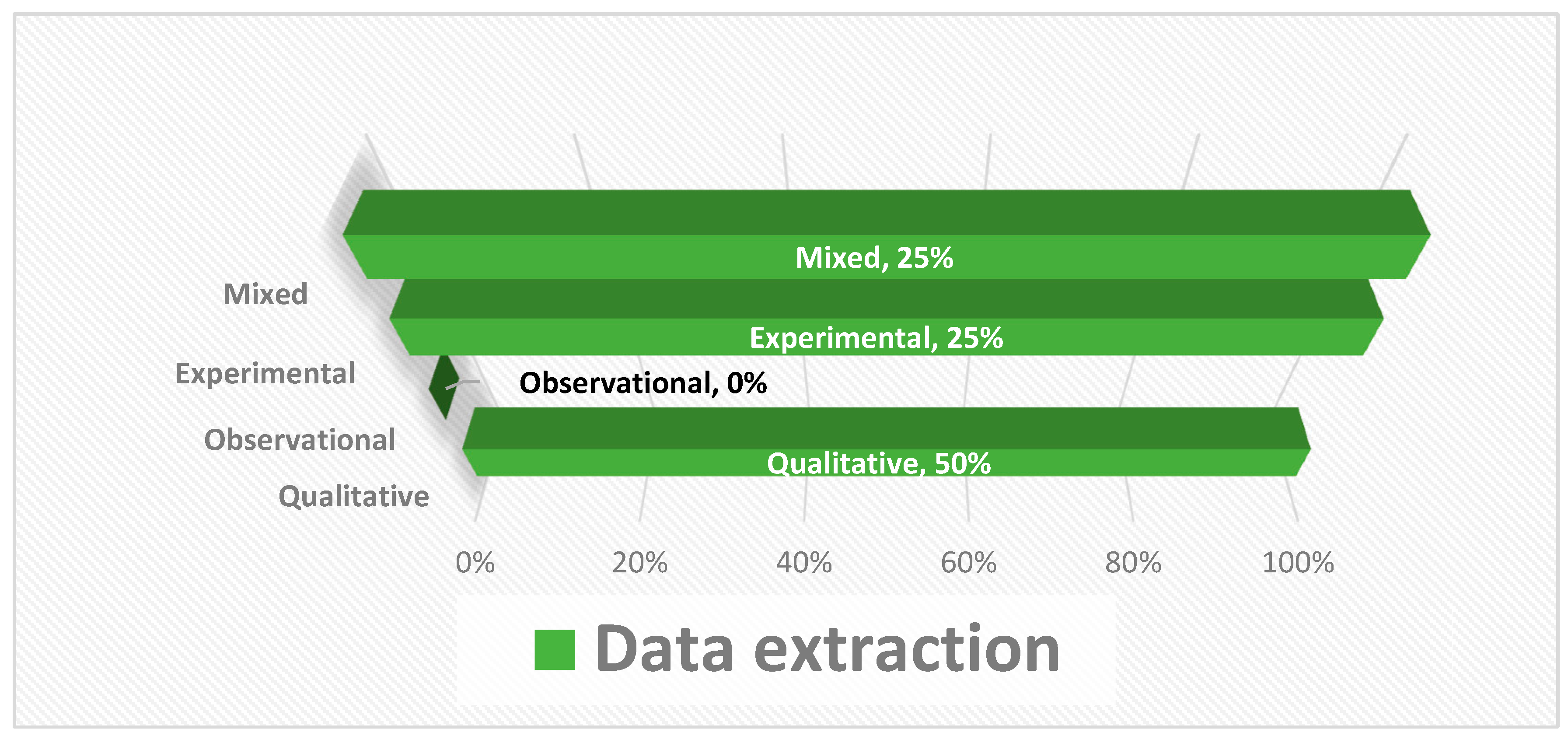
2.4. Study Selection and Quality Assessment
Bias Analysis
- ✓
- Publication bias must be considered, specifically in studies whose results do not coincide with the prescribed interval.
- ✓
- The compilation of the data must be systematic and homogeneous; when there is heterogeneity in the obtained results, it hinders the combination and comparison when different populations are studied.
- ✓
- High heterogeneity may affect the validity of the results of the meta-analysis if included in this study, e.g., a difference between the age of the patients in each population and the results obtained.
3. Results
- Identify the applications of glucocorticoids in oral and maxillofacial surgery in adolescents and adults (Table 3).
- 2.
- Determine the appropriate dose to be administered in oral and maxillofacial surgery in adolescent and adult patients where there is or is not a manifestation of previous pathologies.
- 3.
- Examine the advantages and disadvantages of the different pharmaceutical forms of glucocorticoids.
4. Parenteral Route
- 4.
- Interactions of Glucocorticoids with Other Medications
- 5.
- Describe the effectiveness when glucocorticoids are administered to attend oral manifestations and maxillofacial diseases in adults (16–65 years).
4.1. Population Study in the Range of 18 to 30 Years
4.2. Study of Population over 21 Years
4.3. Population Study in the Range of 16 to 35 Years
4.4. Population Study in the Range of 18 to 25 Years
- 6.
- Study the adverse reactions of glucocorticoids after prolonged use in patients undergoing oral and maxillofacial surgery.
5. Discussion
5.1. Limitations
5.2. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Barnes, P.J. Glucocorticosteroids: Current and future directions. Br. J. Pharmacol. 2009, 163, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-González, M.; Espinosa-Rosales, F. Uso de glucocorticoides sistémicos en Pediatría: Generalidades. Acta Pediátrica México 2016, 37, 349–354. [Google Scholar] [CrossRef]
- Serra, H.A.; Roganovich, J.M.; Rizzo, L.F. Glucocorticoides: Paradigma de medicina traslacional. De lo molecular al uso clínico. Medicina 2012, 72, 158–170. Available online: http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S0025-76802012000200015&lng=es (accessed on 7 April 2023). [PubMed]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of Action of Topical Corticosteroids in Psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef] [PubMed]
- Hodgens, A.; Sharman, T. Corticosteroids. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554612 (accessed on 7 April 2023).
- Bhandage, S.G.; Kurki, M.S.; Sachdeva, G.; Shetty, N.; Kundu, M.; Yadav, A.B. Evaluation of Efficacy of Peri-Operative Administration of Hydrocortisone and Dexamethasone in Prevention of Post-Operative Complications in Oral and Maxillofacial Surgeries; Elsevier: Amsterdam, The Netherlands, 2018; Volume 40, pp. 163–168. Available online: https://www.elsevier.es/es-revista-revista-espanola-cirugia-oral-maxilofacial-300-articulo-evaluation-efficacy-peri-operative-administration-hydrocortisone-S1130055818300042 (accessed on 7 April 2023).
- Olmedo de la Cruz Carranza, H.; Asmat Abanto, Á.S.; Guerrero Guevara, R. Effectiveness of 4 and 8 mg Prophylactic Dexamethasone to Control Post-Surgical Swelling of Impacted Third Molars: A Randomized Parallel-Group Clinical Trial; Elsevier: Amsterdam, The Netherlands, 2013; Volume 35, pp. 157–161. Available online: https://www.revistacirugiaoralymaxilofacial.es/Documentos/ArticulosNew/S113005581300066X.pdf (accessed on 7 April 2023).
- Manrique-Guzmán, J.; Chávez-Reátegui, B.; Manrique-Chávez, J. Glucocorticoides como profiláctico antinflamatorio en cirugía de terceras molares inferiores. Rev. Estomatológica Hered. 2013, 23, 193–199. Available online: https://www.redalyc.org/articulo.oa?id=421539379004 (accessed on 7 April 2023). [CrossRef]
- Chávez-Rimache, L.K.; Rodríguez-Vargas, M.C.; Castro-Rodríguez, Y.; Chumpitaz-Cerrate, V.M. Efecto antinflamatorio de dexametasona y vitaminas B en cirugía de tercer molar. Ensayo clínico aleatorizado. Rev. Esp. Cirug. Oral Maxilofac. 2020, 42, 69–75. Available online: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1130-05582020000200004&lng=es (accessed on 7 April 2023).
- Núñez-Díaz, D.; Chumpitaz-Cerrate, V.; Chávez-Rimache, L.; Santa Cruz, L.G. Comparación de la efectividad antiinflamatoria de dexametasona como terapia prequirúrgica y post-quirúrgica en la cirugía del tercer molar mandibular. Ensayo clínico aleatorizado. J. Oral Res. 2019, 8, 463–470. Available online: http://revistas.udec.cl/index.php/journal_of_oral_research/article/view/1900 (accessed on 7 April 2023). [CrossRef]
- Krishnan, K. Role of corticosteroids in oral and maxillofacial surgery. J. Pharm. Sci. Res. 2018, 10, 208–210. Available online: https://www.proquest.com/scholarly-journals/role-corticosteroids-oral-maxillofacial-surgery/docview/2001045631/se-2 (accessed on 7 April 2023).
- Zandi, M. The Role of Corticosteroids in Today’s Oral and Maxillofacial Surgery; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Boening, K.; Wiland, P.; Shiau, Y.-Y.; Paradowska-Stolarz, A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J. Headache Pain 2015, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, N.; Jayanthi, P.; Rao, U.K.; Ranganathan, K. Oral lichen planus: An update on pathogenesis and treatment. J. Oral Maxillofac. Pathol. 2011, 15, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kuo, R.C.; Lin, H.P.; Sun, A.; Wang, Y.P. Prompt healing of erosive oral lichen planus lesion after combined corticosteroid treatment with locally injected triamcinolone acetonide plus oral prednisolone. J. Formos. Med. Assoc. 2013, 112, 216–220. Available online: https://www.sciencedirect.com/science/article/pii/S0929664612001313 (accessed on 7 April 2023). [CrossRef] [PubMed]
- Venkatesh, E.; Bagewadi, A.; Keluskar, V.; Shetti, A. Role of Corticosteroids in Dentistry. Arch. Dent. Sci. 2010, 1, 3–11. Available online: https://www.researchgate.net/publication/308416384_Role_of_Corticosteroids_in_Dentistry (accessed on 7 April 2023).
- Pinas, L.; Garcia-Garcia, A.; Perez-Sayans, M.; Suarez-Fernandez, R.; Alkhraisat, M.; Anitua, E. The use of topical corticosteroides in the treatment of oral lichen planus in Spain: A national survey. Med. Oral Patol. Oral Cir. Buccal 2017, 22, e264–e269. [Google Scholar] [CrossRef][Green Version]
- Salinas, R.; Alvarez, G.; Alvarez, M.; Ferreira, J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst. Rev. 2002, 7, CD001942. [Google Scholar] [CrossRef]
- Sullivan, F.M.; Swan, I.R.; Donnan, P.T.; Morrison, J.M.; Smith, B.H.; McKinstry, B.; Davenport, R.J.; Vale, L.D.; Ciarkson, J.E.; Hammersley, V.; et al. Early Treatment with Prednisolone or Acyclovir in Bell’s Palsy. N. Engl. J. Med. 2007, 32, 460. [Google Scholar] [CrossRef]
- Belenguer-Guallar, I.; Jimenez-Soriano, Y.; Claramunt-Lozano, A. Treatment of recurrent aphthous stomatitis. A literature review. J. Clin. Exp. Dent. 2014, 6, e168–e174. [Google Scholar] [CrossRef]
- Chiang, C.P.; Chang, J.Y.F.; Wang, Y.P.; Wu, Y.H.; Wu, Y.C.; Sun, A. Recurrent aphthous stomatitis–Etiology, serum autoantibodies, anemia, hematinic deficiencies, and management. J. Formos. Med. Assoc. 2019, 118, 1279–1289. Available online: https://www.sciencedirect.com/science/article/pii/S0929664618307435 (accessed on 7 April 2023). [CrossRef]
- Nair, J.R.; Moots, R.J. Behcet’s disease. Clin. Med. 2017, 17, 71–77. [Google Scholar] [CrossRef]
- Cholera, M.; Chainani-Wu, N. Management of Pemphigus Vulgaris. Adv. Ther. 2016, 33, 910–958. [Google Scholar] [CrossRef] [PubMed]
- Porro, A.M.; Seque, C.A.; Ferreira, M.C.C.; Enokihara, M.M.S. Pemphigus vulgaris. An. Bras. Dermatol. 2019, 94, 264–278. Available online: https://www.scielo.br/j/abd/a/nV5V8fJtZw7kQhhDbzffyVL/abstract/?lang=en (accessed on 7 April 2023).
- Di Lernia, V.; Casanova, D.M.; Goldust, M.; Ricci, C. Pemphigus Vulgaris and Bullous Pemphigoid: Update on Diagnosis and Treatment. Dermatol. Pract. Concept. 2020, 10, e2020050. [Google Scholar] [CrossRef] [PubMed]
- Eming, R.; Sticherling, M.; Hofmann, S.C.; Hunzelmann, N.; Kern, J.S.; Kramer, H.; Pfeiffer, C.; Schuster, V.; Zillikens, D.; Goebeler, M.; et al. S2k guidelines for the treatment of pemphigus vulgaris/foliaceus and bullous pemphigoid. J. Der Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. 2015, 13, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Feliciani, C.; Joly, P.; Jonkman, M.; Zambruno, G.; Zillikens, D.; Ioannides, D.; Kowalewski, C.; Jedlickova, H.; Kárpáti, S.; Marinovic, B.; et al. Management of bullous pemphigoid: The European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br. J. Dermatol. 2015, 172, 867–877. [Google Scholar] [CrossRef]
- Michaels, B. The role of systemic corticosteroid therapy in erythema multiforme major and stevens-johnson syndrome: A review of past and current opinions. J. Clin. Aesthetic Dermatol. 2009, 2, 51–55. [Google Scholar]
- Aggarwal, A.; Suresh, V.; Gupta, B.; Sonthalia, S. Post-herpetic Neuralgia: A Systematic Review of Current Interventional Pain Management Strategies. J. Cutan. Aesthetic Surg. 2020, 13, 265–274. [Google Scholar]
- Johnson, R.W.; Rice, A.S. Postherpetic neuralgia. N. Engl. J. Med. 2014, 371, 1526–1533. [Google Scholar] [CrossRef]
- Elhag, H.A.O.; Babikir, M.H.; Tarakji, B. Advantages and disadvantages of surgical and non-surgical treatment of central giant-cell granuloma: A Review of literature. Int. J. Contemp. Dent. Med. Rev. 2017, 22–29. Available online: https://www.researchgate.net/profile/Kumar-Nilesh/publication/315654032_Giant_Cell_Lesion_and_Central_Giant_Cell_Granulo-ma_of_Jaw_A_Brief_Review/links/58d811b1a6fdcc1baeb8eab1/Giant-Cell-Lesion-and-Central-Giant-Cell-Granuloma-of-Jaw-A-Brief-Review.pdf (accessed on 7 April 2023).
- Niedzielska, I.; Bielecki, M.; Bąk, M.; Dziuk, B.; Niedzielski, D. Bony Canal Method of Dexamethasone Injections in Aggressive Form of Central Giant Cell Granuloma—Case Series. Medicina 2023, 59, 250. [Google Scholar] [CrossRef]
- Kent, S.; Hennedige, A.; McDonald, C.; Henry, A.; Dawoud, B.; Kulkarni, R.; Logan, G.; Gilbert, K.; Exely, R.; Basyuni, S.; et al. Systematic review of the role of corticosteroids in cervicofacial infections. Br. J. Oral Maxillofac. Surg. 2019, 57, 196–206. [Google Scholar] [PubMed]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Asokan, G.S. Corticosteroids in oral diseases. J. Indian Dent. Assoc. Trivandrum Branch 2012, 9–13. Available online: http://www.trivandrumdentaljournal.org/Trivandrum%20Dental%20Vol%203%20Issue%201.pdf#page=11 (accessed on 7 April 2023).
- Brenner, G.M.; Stevens, C.W. Farmacología Básica; Brenner, G.M., Stevens, C.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 7–9. [Google Scholar]
- York, P. Design of dosage forms. In The Design and Manufacture of Medicines, 6th ed.; Aulton, M.; Taylor, K., Translators; Elsevier: Exeter, UK, 2022; pp. 1–7. [Google Scholar]
- Galofré, J.C. Manejo de los corticoides en la práctica clínica. Rev. Med. Univ. Navar. 2017, 53, 9–18. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4052587/ (accessed on 7 April 2023).
- Grennan, D.; Wang, S. Steroid Side Effects. JAMA 2019, 322, 282. [Google Scholar] [CrossRef]
- Pérez, S.; Saiz, A.; Munoz, J.; Bazaco, V.; Baron, D.; Ruiz, M. Vías y procedimientos de administración de fármacos. Ocronos 2022, 5, 29. Available online: https://revistamedica.com/?s=vias+y+procedimientos++de+administracion+farmacos (accessed on 7 April 2023).
- Verma, P.; Thakur, A.S.; Deshmukh, K.; Jha, A.K.; Verma, S. Routes of drug administration. Int. J. Pharm. Stud. Res. 2010, 1, 54–59. Available online: https://romanpub.com/resources/ijpsr%20v11-2020-7.pdf (accessed on 7 April 2023).
- Wolverton, S.E. Systemic corticosteroids. Compr. Dermatol. Drug Ther. 2012, 3, 143–168. [Google Scholar]
- Medline. Available online: https://www.mayoclinic.org/steroids/ART-20045692?p=1 (accessed on 7 April 2023).
- Bhanot, R.; Mago, J. Corticosteroids in dentistry. Indian J. Dent. Sci. 2016, 8, 252. [Google Scholar] [CrossRef]
- Prete, A.; Bancos, I. Glucocorticoid induced adrenal insufficiency. BMJ 2021, 374, N1380. Available online: https://tangsclinical.com/wp-content/uploads/2021/07Glucocorticoid-induced-adrenal-insufficiency-BMJ.pdf (accessed on 7 April 2023). [CrossRef] [PubMed]
- Hyle, E.P.; Wood, B.R.; Backman, E.S.; Noubary, F.; Hwang, J.; Lu, Z.; Losina, E.; Walensky, R.P.; Gandhi, R.T. High Frequency of Hypothalamic-Pituitary-Adrenal Axis Dysfunction After Local Corticosteroid Injection in HIV-Infected Patients on Protease Inhibitor Therapy. Am. J. Ther. 2013, 63, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Saberi, P.; Phengrasamy, T.; Nguyen, D. Inhaled corticosteroid use in HIV-positive individuals taking protease inhibitors: A review of pharmacokinetics, case reports and clinical management. HIV Med. 2013, 14, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.D.; Hadigan, C.; Mcmanus, M.R.; Chairez, C.R.; Nieman, L.K.; Pau, A.K.; Alfaro, R.M.; Kovacs, J.A.; Calderon, M.M.; Penzak, S.R. Influence of low-dose ritonavir with and without darunavir on the pharmacokinetics and pharmacodynamics of inhaled beclomethasone. Am. J. Ther. 2013, 63, 355–361. [Google Scholar] [CrossRef]
- Drummond, M.B.; Kirk, G.D. HIV-associated obstructive lung diseases: Insights and implications for the clinician. Lancet Respir. Med. 2014, 2, 583–592. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef]
- Fan, L.; Sweet, D.R.; Fan, E.K.; Prosdocimo, D.A.; Madera, A.; Jiang, Z.; Padmanabhan, R.; Haldar, S.M.; Vinayachandran, V.; Jain, M.K. Transcription factors KLF15 and PPARδ cooperatively orchestrate genome-wide regulation of lipid metabolism in skeletal muscle. J. Biol. Chem. 2022, 298, 101926. [Google Scholar] [CrossRef]
- Jo, J.-R.; Lee, S.-E.; An, S.; Nedumaran, B.; Ghosh, S.; Park, K.-G.; Kim, Y.D. Gluconeogenic signals regulate hepcidin gene expression via a CRBN-KLF15 axis. BMB Rep. 2021, 54, 221–226. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Respond to the objectives set. | Not related to the objectives. |
| Study types: observational, randomized clinical trials. | Full texts not available. |
| Articles published in the last 10–15 years. | Articles that do not meet the inclusion criteria or with little proven scientific evidence (PEDro scale less than 7). |
| Articles are open access, free, and complete. | |
| Articles published in English, Spanish, Portuguese. | |
| Population between 10 and 65 years old/any race and sex. | |
| Valued with the PEDro scale higher than 7. |
| 1st Author, Year | Bias from Random Sequence | Bias Allocation Concealment | Bias Blinding Participant and Personnel | Bias Blinding Outcome | Incomplete Outcome Data | Selective Report | Other Bias |
|---|---|---|---|---|---|---|---|
| Bhandage 2018 [7] | + | + | ? | + | + | + | + |
| De la Cruz Carranza 2013 [8] | + | + | - | + | + | + | + |
| Manriquez-Guzman 2013 [9] | + | + | - | + | + | + | + |
| Chavez-Rimache 2020 [10] | + | + | + | + | + | + | + |
| Nunez-Dias 2019 [11] | + | + | + | + | + | + | + |
| Pathology | Drug | Route of Administration |
|---|---|---|
| Temporomandibular joint disorders. | 10 mg/day methylprednisolone, betamethasone acetate, triamcinolone acetonide, and hydrocortisone. | Intra-articular route. Limit application to 4 times a year. |
| Oral lichen planus. | Triamcinolone acetonide 0.1%, betamethasone sodium phosphate 0.1–0.05%, clobetasol 0.05%. | Topical route. Two or three times a day if a lesion is present. |
| Bell’s palsy. | Prednisolone 1 mg/kg/day | Orally. 7–10 days. |
| Recurrent aphthous stomatitis. | Hydrocortisone hemisuccinate 2.5 mg, triamcinolone acetonide 0.1%, dexamethasone 0.5 mg. | Topical route on the lesion two or three times a day and before sleeping (gel or paste). |
| Behçet’s disease. | Immunosuppressive therapy. In acute phase, prednisone 40–60 mg/day. | Orally. |
| Pemphigus diseases. | Prednisone 0.5–1.5 mg/kg/day or 10–40 mg highly potent topical corticosteroids. | Orally and topically, reducing or increasing the dose according to the severity of the lesion. |
| Erythema multiforme. | Clobetasol propionate rinses in aqueous solution. In case of aggravation of the lesion, prednisone 40–80 mg/kg/day, methylprednisolone 1 mg/kg/day (3 days). | Topical route. Mouthwashes. Oral route in case of aggravation (1–2 weeks and reduce dose). |
| Postherpetic neuralgia. | Methylprednisolone 40 mg/10 day. | Intramuscular route. |
| Central giant-cell granuloma. | Dexamethasone 0.5% (3 mg/mL/0) injections once a week for six weeks. | Intramuscular route. |
| Drug | Usual Dose |
|---|---|
| Hydrocortisone (IV, IM, topical) | 20 mg–240 mg/day |
| Prednisone (orally) | 5 mg–60 mg/day |
| Prednisolone (orally) | 5 mg–60 mg/day |
| Methylprednisolone (orally, IV, IM) | 10 mg–0 mg/day |
| Triamcinolone (topical, orally) | 10 mg–60 mg/day or 0.1 mg–0.3 mg |
| Betamethasone (IV, IM, orally) | 0.6 mg–7.2 mg/day |
| Dexamethasone (IV, IM, orally) | 0.75 mg–9.0 mg/day |
| Albendazole: GCs reduce the metabolism of antiparasitic agents, favoring gastrointestinal and hepatic toxicity. |
| Antacids: reduce GC absorption. |
| Antifungal azoles: increase plasma levels of GCs, causing adverse effects. |
| Barbiturates: reduce GC catabolism and reduce activation of the prodrugs prednisone and methylprednisolone. |
| Thyroid hormones: accelerate GC catabolism, which leads to loss of efficacy. |
| Progestogens and oral contraceptives: reduce GC catabolism increasing the effect and promoting toxicity. |
| GCs are CYP3A4 inducers, which can reduce the efficacy of some drugs by increasing their catabolism, e.g., benzodiazepines, tretinoin, quetiapine, statins, tyrosine kinase inhibitors. |
| Article | Purpose of the Study | Intervention | Results | Conclusion |
|---|---|---|---|---|
| Evaluation of efficacy of peri-operative administration of hydrocortisone and dexamethasone in prevention of post-operative complication in oral and maxillofacial surgeries. | Examine the roles of intraoperative administration of hydrocortisone and postoperative dexamethasone in minimizing postoperative complications after major surgeries of the oral cavity under general anesthesia. | N = 20 patients (25–65 years). General anesthesia (intubation and extubating) was used. Procedures included maxillary, mandibular, and zygomatic-maxillary complex fractures (trauma) and surgeries to treat pathologies such as keratocystic odontogenic tumors. | Intervention: single IP dose of hydrocortisone. Result: 2nd postsurgical day: 70% pain reduction. Fourth postsurgical day pain reduction of 97% according to numerical scale (EN), accompanied by 12 mm reduction in edema. No patient developed ADRs, such as nausea or vomiting, at the postoperative level. | Administration of a single IP dose of hydrocortisone and adjusted postoperative dexamethasone helps combat most postoperative complications after surgical interventions; therefore, it is an effective and safe drug. |
| Bhandage et al. 2018 [7]. | ||||
| Article | Purpose of the Study | Intervention | Results | Conclusion |
|---|---|---|---|---|
| Effectiveness of 4 and 8 mg prophylactic dexamethasone to control post-surgical swelling of impacted third molars: A randomized parallel-group clinical trial. | To verify the effectiveness of prophylactic dexamethasone orally (PO) 8 mg with 4 mg to control postsurgery edema of impacted third molars. | N = 66 patients (18–30 years). Lower third molar included asymptomatic and moderate level of difficulty according to the classification of Koerner et al. | Intervention: 27 received 8 mg PO prophylactic dexamethasone and 27 4 mg. Result: 8 mg dexamethasone was more effective than 4 mg dexamethasone. | 8 mg PO prophylactic dexamethasone is more effective than 4 mg for controlling postsurgery edema of third molars. |
| De la Cruz Carranza et al. 2013 [8]. | ||||
| Article | Purpose of the Study | Intervention | Results | Conclusion |
|---|---|---|---|---|
| Glucocorticoids as a prophylactic anti-inflammatory in inferior third molar surgery. | To administer glucocorticoid medication (dexamethasone 8 mg via IM) 1 h before the treatment of complex exodontia via intramuscular route in one group and not to the other group, later evaluating the presence of severe acute inflammation. | N = 116 patients (21–45 years female/male). Randomly divided into two groups, only one received glucocorticoid medication before treatment. | Intervention: dose of dexamethasone 8 mg IM one hour before treatment. Result: it was found that 92% of the group that did not receive previous medication presented acute pain during the first 48 h, and 82% and 80% presented signs of edema and trismus, respectively. In contrast, 12%, 4%, and 2% of patients who received prior medication presented signs and symptoms of acute pain, edema, and lockjaw, respectively. | The appearance of signs and symptoms of severe acute inflammation was greater in the group that did not receive glucocorticoid medication before the intervention. |
| Manrique-Guzmán et al. 2013 [9]. | ||||
| Article | Purpose of the Study | Intervention | Results | Conclusion |
|---|---|---|---|---|
| Anti-inflammatory effect of dexamethasone and B vitamins in third molar surgery. Randomized clinical trial. | To analyze the anti-inflammatory effect of the preoperative administration of the combination of dexamethasone with B vitamins in mandibular third molar surgeries. | N = 54 patients (18–25 years). Control group was administered 4 mg of dexamethasone and the experimental group the combination of 4 mg of dexamethasone with vitamins B1, B6, and B12: via IM before surgery. Pain was evaluated using the visual analogue scale (VAS). | It was shown that the greatest magnitude of pain appeared at 24 h, being significantly lower in the experimental group. Facial swelling increased progressively until the 3rd day, with no significant difference between the groups. | A significantly greater analgesic activity and a significantly lower total consumption of analgesics were evidenced in the group that used dexamethasone and group B vitamins as an adjuvant. |
| Chávez-Rimache et al. 2020 [10]. | ||||
| Article | Purpose of the Study | Intervention | Results | Conclusion |
|---|---|---|---|---|
| Comparison of the anti-inflammatory effectiveness of dexamethasone as pre-surgical and post-surgical therapy in mandibular third molar surgery: A randomized clinical trial. | To compare the anti-inflammatory effectiveness of dexamethasone as pre-surgical and postsurgical therapy in mandibular third molar surgery. | N = 60 patients (16 to 35 years). Mandibular third molar extraction. Group A received 4 mg of dexamethasone intramuscularly pre-surgery, and group B received the same medication postsurgery. Pain intensity was evaluated using the numerical scale (EN). | The values of facial edema were lower in group A at 60 min than in group B. Regarding pain, the highest intensity was perceived at 6 h in both groups, with no significant difference between them. | Preoperative administration of dexamethasone produced a significantly greater reduction in facial edema following mandibular third molar surgery. |
| Nunez-Diaz et al. 2019 [11]. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nils, H.J.; Arce Recatala, C.; Castano, A.; Ribas, D.; Flores-Fraile, J. Efficacy/Safety of the Use of Glucocorticoids in Oral and Maxillofacial Surgery. Dent. J. 2023, 11, 239. https://doi.org/10.3390/dj11100239
Nils HJ, Arce Recatala C, Castano A, Ribas D, Flores-Fraile J. Efficacy/Safety of the Use of Glucocorticoids in Oral and Maxillofacial Surgery. Dentistry Journal. 2023; 11(10):239. https://doi.org/10.3390/dj11100239
Chicago/Turabian StyleNils, Heilyn Joanna, Cristina Arce Recatala, Antonio Castano, David Ribas, and Javier Flores-Fraile. 2023. "Efficacy/Safety of the Use of Glucocorticoids in Oral and Maxillofacial Surgery" Dentistry Journal 11, no. 10: 239. https://doi.org/10.3390/dj11100239
APA StyleNils, H. J., Arce Recatala, C., Castano, A., Ribas, D., & Flores-Fraile, J. (2023). Efficacy/Safety of the Use of Glucocorticoids in Oral and Maxillofacial Surgery. Dentistry Journal, 11(10), 239. https://doi.org/10.3390/dj11100239