Abstract
Calcium (Ca2+) and magnesium (Mg2+) are essential for cellular function. The kidneys play an important role in maintaining the homeostasis of these cations. Their reabsorption along the nephron is dependent on distinct trans- and paracellular pathways and is coupled to the transport of other electrolytes. Notably, sodium (Na+) transport establishes an electrochemical gradient to drive Ca2+ and Mg2+ reabsorption. Consequently, alterations in renal Na+ handling, under pathophysiological conditions or pharmacological manipulations, can have major effects on Ca2+ and Mg2+ transport. One such condition is the administration of diuretics, which are used to treat a large range of clinical conditions, but most commonly for the management of blood pressure and fluid balance. While the pharmacological targets of diuretics typically directly mediate Na+ transport, they also indirectly affect renal Ca2+ and Mg2+ handling through alterations in the electrochemical gradient. To investigate renal Ca2+ and Mg2 handling and how those processes are affected by diuretic treatment, we have developed computational models of electrolyte transport along the nephrons. Model simulations indicate that along the proximal tubule and thick ascending limb, the transport of Ca2+ and Mg2+ occurs in parallel with Na+, but those processes are dissociated along the distal convoluted tubule. We also simulated the effects of acute administration of loop, thiazide, and K-sparing diuretics. The model predicted significantly increased Ca2+ and Mg2+ excretions and significantly decreased Ca2+ and Mg2+ excretions on treatment with loop and K-sparing diuretics, respectively. Treatment with thiazide diuretics significantly decreased Ca2+ excretion, but there was no significant alteration in Mg2+ excretion. The present models can be used to conduct in silico studies on how the kidney adapts to alterations in Ca2+ and Mg2+ homeostasis during various physiological and pathophysiological conditions, such as pregnancy, diabetes, and chronic kidney disease.
1. Introduction
The divalent cations, Ca2+ and Mg2+, are important for various physiological processes. About 99% of the body’s Ca2+ is stored in bones, where it forms a calcium-phosphate compound called hydroxyapatite [1]. The remaining 1% of body calcium plays an important role in various other physiological processes, such as cell signaling, both skeletal and smooth muscle contraction, and blood clotting [1]. Mg2+ plays a pivotal role in energy-demanding metabolic reactions, protein synthesis, ensuring membrane integrity, facilitating nervous tissue conduction, promoting neuromuscular excitability, regulating muscle contraction, influencing hormone secretion, and participating in intermediary metabolism. Nearly 99% of the body’s Mg2+ is distributed within cells or stored in bone, with only a small fraction in circulation [2]. Tight regulation of the serum Ca2+ and Mg2+ concentrations is essential since too much or too little Ca2+ or Mg2+ can have dangerous, potentially fatal consequences. To maintain Ca2+ and Mg2+ balance, it is crucial to regulate the fluxes of Ca2+ and Mg2+ among the primary organs involved in their regulation, namely the intestine, bone, and kidneys.
The kidneys play an important role in maintaining Mg2+ and Ca2+ homeostasis. The majority, ~60–70% of the filtered Ca2+, is reabsorbed along the proximal tubule through the paracellular pathway [3]. By contrast, paracellular Mg2+ permeability in the proximal tubule is very low, and hence only 15–25% of the filtered Mg2+ is reabsorbed along this segment [3]. The majority of the filtered Mg2+ is reabsorbed along the cortical thick ascending limb (60–70%) paracellularly [3]. The paracellular fractional reabsorption of Ca2+ along the thick ascending limb is ~15–25% [3]. The distal convoluted tubule is the final segment that reabsorbs Mg2+; hence, it plays an important role in fine-tuning urinary Mg2+ excretion. Approximately 5–10% of the filtered Mg2+ is reabsorbed transcellularly along the distal convoluted tubule, mediated by the transient receptor potential melastatin 6/7 (TRPM6/7) heteromeric complex on the apical membrane and the Na+/Mg2+ exchanger on the basolateral membrane [3]. Approximately 5–10% of the filtered Ca2+ is reabsorbed transcellularly along the distal convoluted tubule and connecting tubule, mediated by the transient receptor potential vanilloid 5 (TRPV5) on the apical membrane, the Na+/Ca2+ exchanger (NCX1), and plasma membrane Ca2+-ATPase (PMCA) on the basolateral membrane [3]. Finally, ~2–5% of the filtered Mg2+ and Ca2+ are excreted through urine [3].
Our understanding of Ca2+ and Mg2+ handling within different segments of the nephron has been greatly advanced through micropuncture and microperfusion studies in rodent nephrons [4,5]. Furthermore, recent genetic studies have expanded our knowledge about the protein mediators of Ca2+ and Mg2+ transport [6]. Despite these advances, our understanding of the renal handling of these electrolytes remains incomplete. What fraction of the renal reabsorption goes through the transcellular versus paracellular pathway? To what extent is the renal transport in each nephron segment coupled to the transport of other electrolytes, e.g., Na+, K+, and Cl−? To answer these questions, we developed a detailed computational model of epithelial transport of electrolytes and water along the nephrons in a male rat kidney and conducted simulations to predict the renal transport of Ca2+ and Mg2+ as well as other electrolytes and water under different physiological conditions.
Besides electrolyte and fluid homeostasis, the kidney also plays an essential role in maintaining normal blood pressure. For the management of blood pressure and fluid balance, diuretics are commonly prescribed. Although the pharmacological targets of diuretics directly affect Na+ transport, they also indirectly affect renal Mg2+ and Ca2+ reabsorption through changes in the electrochemical gradient. How is renal Ca2+ and Mg2+ transport affected by the administration of diuretics? To answer this question, we simulate the effect of acute administration of three classes of diuretics—loop; thiazide; and K-sparing diuretics—on renal Mg2+ and Ca2+ transport and excretion.
2. Materials and Methods
We have previously developed epithelial cell-based computational models of transporter-mediated solute and water transport along the nephron of a rat kidney [7,8,9,10], focusing on the renal handling of Na+, K+, Ca2+, glucose, and water in physiological and pathophysiological conditions. The superficial nephron model includes the proximal tubule, short descending limb, thick ascending limb, distal convoluted tubule, connecting tubule, and collecting duct segments. Each nephron segment is represented as a tubule lined by a layer of epithelial cells. The model tracks the transport of the following 17 solutes: Na+, K+, Cl−, HCO3−, H2CO3, CO2, NH3, NH4+, HPO42−, H2PO4−, H+, HCO2−, H2CO2, urea, glucose, Ca2+, and Mg2+. The segment and cell type determine the type and abundance of transporters found on the apical and basolateral membranes of the cell. Solutes and water may be transported across the epithelium by either moving across the apical and basolateral membranes in the transcellular pathway, mediated by specialized membrane transporters or channels, or via the paracellular pathway between neighboring cells. A schematic diagram for the model nephrons is shown in Figure 1.
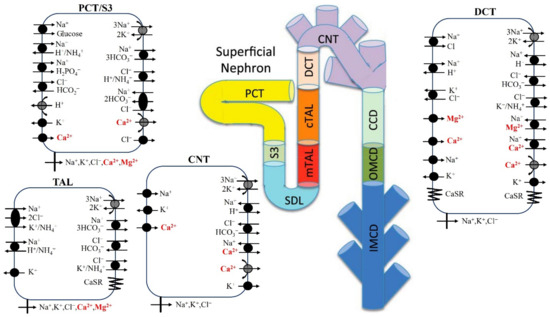
Figure 1.
Model diagram of epithelial transport of Ca2+ and Mg2+ and selected electrolytes along the superficial nephron. Mg2+ transport occurs along the proximal tubule (proximal convoluted tubule, PCT, and S3), cortical thick ascending limb (cTAL), and distal convoluted tubule (DCT). Ca2+ transport occurs along the proximal tubule (proximal convoluted tubule, PCT, and S3), medullary/cortical thick ascending limb (mTAL/cTAL), distal convoluted tubule (DCT), and connecting tubule (CNT). Only the major Na+, K+, Cl−, Ca2+, and Mg2+ transporters are shown. PCT, proximal convoluted tubule; SDL, short descending limb; mTAL, medullary thick ascending limb; limb; CNT, connecting tubule; CCD, cortical collecting duct; OMCD, outer-medullary collecting duct; IMCD, inner-medullary collecting duct.
The model is defined by a large system of coupled differential and algebraic equations that describe mass conservation and determine transmembrane and paracellular fluxes [11]. The model predicts luminal fluid flow, hydrostatic pressure, membrane potential, luminal and cytosolic solute concentrations, transcellular and paracellular fluxes, urine volume, and urinary excretion rates of model solutes.
Below, we summarize a model representation of Mg2+ transport along the proximal tubule, cortical thick ascending limb, and distal convoluted tubule. Model parameters that describe Mg2+ transport are given in Table 1. The analogous model description and parameter for Ca2+ transport can be found in Ref. [10]. Additional model parameters can be found in Ref. [12].
2.1. Mg2+ Transport along the Proximal Tubule
The proximal tubule reabsorbs 15–25% of the filtered Mg2+ load through the paracellular pathway, which is mediated by claudin-2 and -12 [13] and is driven by the favorable electrochemical gradient established by Na+/H+ exchanger 3 (NHE3)-mediated Na+ reabsorption [5,14,15]. Paracellular electro-diffusive Mg2+ flux () is given by
where the superscripts L and I denote lumen and lateral intercellular space (LIS), respectively; denotes the permeability of Mg2+ at the lumen and LIS interface; ; is the valence of Mg2+ (+2); and denote Mg2+ concentrations in the lumen and LIS, respectively; and denote the luminal and LIS membrane potentials, respectively; RT = 2.57 J/mmol; and F = 96.5 C/mmol represents Faraday’s constant.
2.2. Mg2+ Transport along the Thick Ascending Limb
The medullary thick ascending limb has negligible Mg2+ reabsorption [15,16,17]. In contrast, the cortical thick ascending limb reabsorbs 60–70% of the filtered Mg2+ via the paracellular route. That flux is mediated by claudins 16 and 19 [18], and is driven by the electrochemical gradient established by Na+-K+-Cl− cotransporter 2 (NKCC2)-mediated Na+ transport [9,14]. Paracellular Mg2+ transport in the cortical thick ascending limb is represented by an expression analogous to Equation (1), with the superscript “PT” replaced by “cTAL”.
2.3. Mg2+ Transport along the Distal Convoluted Tubule
Unlike the proximal tubule and cortical thick ascending limb, where Mg2+ transport proceeds passively via the paracelluar route, the reabsorptive process along the distal convoluted tubule is active and transcellular and is mediated by the TRPM6 and TRPM7 (TRPM6/7) heteromeric channels, expressed on the apical membranes [19]. Mg2+ flux through TRPM6/7 is given by
and describe the effects of intracellular Mg2+ concentration () and extracellular pH on TRPM6/7 [20], and are given by
where denotes the single channel conductance of TRPM6/7 channel at pH 7.4, denotes the luminal fluid pH, and denotes the luminal fluid pH for half-maximal conductance.
Mg2+ efflux through the basolateral membrane is assumed to be mediated by an Na+/Mg2+ exchanger [19,21], given by
where denotes the maximum Mg2+ flux through the Na+/Mg2+ exchanger, and the f terms represent regulation by extracellular Na+, , intracellular Na+, , extracellular Mg2+, , and intracellular Mg2+, . In these expressions, , , , and denote the Michaelis-Menten constants.
2.4. Calcium-Sensing Receptor
The calcium-sensing receptor (CaSR) regulates not only Ca2+ and Mg2+ reabsorption in the kidneys but other electrolytes and water as well by modifying transporter activities. Ca2+ is the primary ligand for activating CaSR. At equimolar concentrations, Mg2+ is 1/2 to 2/3 as potent as Ca2+ in activating CaSR [22,23]. We model the effect of CaSR on a given parameter ( may denote paracellular permeability, NKCC2 activity, ROMK activity, or NCC activity; see below) with the following expression:
where is the value of in the absence of the effect of CaSR, and denote the concentration of Ca2+ and Mg2+ in the luminal (i = L) or interstitial (i = S) fluid, and = 1.25 mM and = 2.5 mM represent the half-maximal concentrations for Ca2+ and Mg2+ [24], respectively. CaSR is ubiquitously expressed in the kidney both along the apical and basolateral membranes, with its highest expression being at the basolateral membrane of the cortical thick ascending limb [25]. Hence, we represent for (i) paracellular permeability, NKCC2 activity, and renal outer-medullary potassium channel (ROMK) in the thick ascending limb, (ii) Na+-Cl− cotransporter (NCC) activity in the distal convoluted tubule, (iii) H+-ATPase flux in outer-medullary collecting duct type A cells, and (iv) water permeability in the inner-medullary collecting duct. The parameters and are negative if CaSR has an inhibitory effect on and positive otherwise. Since the effect of Mg2+ on CaSR activation is ~50–66% of that of Ca2+, we set = 0.6. The values for and for each of the segments are given in Table 1.

Table 1.
Mg2+-specific parameters for all the segments along the superficial nephron. Values marked (*) are adjusted. PT, proximal tubule; TAL, thick ascending limb; DCT, distal convoluted tubule; CD, collecting duct; OMCD, outer-medullary collecting duct; IMCD, inner-medullary collecting duct.
Table 1.
Mg2+-specific parameters for all the segments along the superficial nephron. Values marked (*) are adjusted. PT, proximal tubule; TAL, thick ascending limb; DCT, distal convoluted tubule; CD, collecting duct; OMCD, outer-medullary collecting duct; IMCD, inner-medullary collecting duct.
| Parameter | Value |
|---|---|
| PT | |
| Tight junction permeability to Mg2+ at the lumen-LIS interface () | 1.1 × 10−5 cm/s [26] |
| Reflection coefficient of tight junction to Mg2+ | 0.89 (*) |
| cTAL | |
| Tight junction permeability at the lumen-LIS interface in the absence of Mg2+ () | 38 × 10−5 cm/s (*) |
| Maximum half concentration of Ca2+ () | 1.25 mM [24] |
| Maximum half concentration of Mg2+ () | 2.5 mM [24] |
| Hill function coefficient, n | 4 [24] |
| Inhibitory coefficient of Ca2+ on tight junction permeability () | −4/7 [27] |
| Inhibitory coefficient of Mg2+ on tight junction permeability () | −0.34 (*) |
| Inhibitory coefficient of Ca2+ on NKCC2 activity () | −0.4 [28] |
| Inhibitory coefficient of Mg2+ on NKCC2 activity () | −0.24 (*) |
| Inhibitory coefficient of Ca2+ on ROMK activity () | −0.8 [29,30] |
| Inhibitory coefficient of Mg2+ on ROMK activity () | −0.48 (*) |
| DCT | |
| TRPM6/7 channel density () | 26 × 104 cm-2 (*) |
| Single channel conductance of TRPM6/7 at pH 7.4 () | 56.6 pS [20] |
| Luminal pH for half-maximal conductance of TRPM6/7 () | 5.5 [20] |
| Maximum Mg2+ flux through Na+/Mg2+ exchanger () | 8.6 × 10−9 mmol/cm2/s (*) |
| Intracellular Mg2+ half-saturation constant () | 3.59 M [19,21] |
| Extracellular Mg2+ half-saturation constant () | 1.3 mM [19,21] |
| Intracellular Na+ half-saturation constant () | 12.29 mM [19,21] |
| Extracellular Na+ half-saturation constant () | 87.5 mM [19,21] |
| Excitatory coefficient of Ca2+ on NCC activity () | 0.5 [31] |
| Excitatory coefficient of Mg2+ on NCC activity () | 0.3 (*) |
| CD | |
| Ca2+ promoting coefficient for apical HATPase activity of type A OMCD cells () | 2 [27] |
| Mg2+ promoting coefficient for apical HATPase activity of type A OMCD cells () | 1.2 (*) |
| Ca2+ inhibitory coefficient for apical water permeability of IMCD cells () | −3/8 [27] |
| Mg2+ inhibitory coefficient for apical water permeability of IMCD cells () | −0.225 (*) |
3. Results
3.1. Baseline Results
Using rat parameters, we computed the model’s luminal fluid flow, luminal fluid solute concentrations, cytosolic solute concentrations, membrane potential, and fluxes. Figure 2 shows the predicted segmental delivery, transport, and luminal fluid concentration of Mg2+ and Ca2+ along the nephron. Results for other electrolytes and water can be found in Ref. [32].
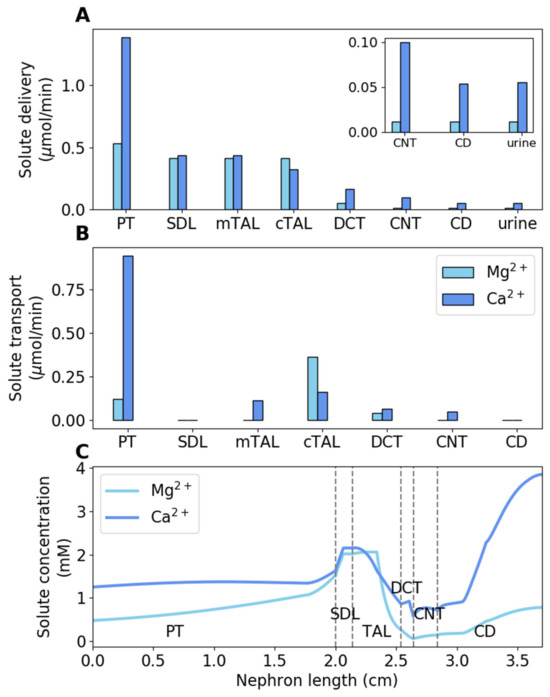
Figure 2.
Baseline results. (A) Delivery of Mg2+ and Ca2+ to key nephron segments in male rats, given per kidney. (B) Mg2+ and Ca2+ transepithelial transport along key nephron segments in male rats, given per kidney. (C) Luminal Mg2+ and Ca2+ concentrations along key nephron segments in male rats. PT, proximal tubule; SDL, short descending limb; mTAL, medullary thick ascending limb; limb; cTAL, cortical thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct.
The majority of the filtered Ca2+ is reabsorbed along the proximal tubule, accounting for 68% of the filtered load; by contrast, Mg2+ reabsorption along this segment accounts for only 22% of the filtered load. Since Mg2+ reabsorption is low along the proximal tubule, Mg2+ concentration increases by ~3-fold [4]. The majority of the overall Mg2+ transport occurs downstream along the cortical thick ascending limb (68% of the filtered load), where the lumen-positive membrane potential drives Mg2+ reabsorption via the paracellular pathway. The fractional reabsorption of Ca2+ along the medullary and cortical thick ascending limb is 20%. The final nephron segment that transports Mg2+ is the distal convoluted tubule, where 6.6% of the filtered Mg2+ is reabsorbed. The fractional reabsorption of Ca2+ along the distal convoluted tubule and connecting tubule is 7.9%. Finally, fractional urinary Mg2+ and Ca2+ excretions are 3.2% and 3.9%, respectively.
3.2. Effect of Loop Diuretics
Loop diuretics inhibit NKCC2, which is expressed on the apical membrane of the thick ascending limb. We simulated the effect of acute administration of loop diuretics by inhibiting NKCC2 activity by 70%. We assumed that the NKCC2 inhibitor was administered for long enough to significantly impair the kidney’s ability to generate an axial osmolality gradient. The cortical interstitial concentrations were assumed to remain unchanged. Since the concentrating mechanism of the outer medulla is significantly impaired following complete NKCC2 inhibition, the interstitial concentrations of Mg2+ and Ca2+ at the outer-inner medullary boundary are lowered to 0.77 mM (from a baseline value of 0.96 mM) and 2.0 mM (from a baseline value of 2.5 mM), respectively. At the papillary tip, the interstitial concentrations of Mg2+ and Ca2+ are reduced to 1.0 mM (from 1.54 mM) and 2.62 mM (from 4.0 mM), respectively. For changes in the interstitial concentrations of Na+, K+, Cl−, and urea, refer to [9].
The predicted Mg2+ and Ca2+ transport along the thick ascending limb and distal tubules and urinary Mg2+ and Ca2+ excretions following NKCC2 inhibition in male rats are shown in Figure 3. Our NKCC2 inhibition simulations predicted the fractional Mg2+ reabsorption along the cortical thick ascending limb to decrease to 57% from the baseline fractional reabsorption of 69% (Figure 3). Administration of furosemide, a loop diuretic, to male mice increased TRPM6 mRNA expression by 30% [33]. Our model simulations predicted a 68% increase in TRPM6/7 channel activity to account for the 240% increase in Mg2+ excretion in male rats undergoing furosemide treatment [34]. Fractional Ca2+ reabsorption along the thick ascending limb decreased by 17% following NKCC2 inhibition (Figure 3). This resulted in urinary Ca2+ excretion increasing to 236% of the baseline excretion value (Figure 3).
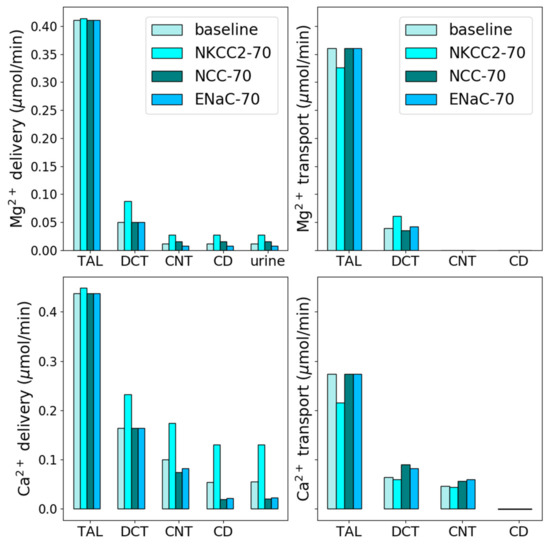
Figure 3.
Effects of diuretic treatment. Delivery and transport of Mg2+ and Ca2+ along key nephron segments in male rats under normal conditions and 70% inhibition of NKCC2, NCC, and ENaC. The values are given per kidney. TAL, thick ascending limb; limb; DCT, distal convoluted tubule; CNT, connecting tubule; CD, collecting duct.
3.3. Thiazide Diuretics
Thiazide diuretics inhibit NCC, which is expressed along the apical membrane of the distal convoluted tubule. We simulated the effect of acute administration of thiazide diuretics by inhibiting NCC activity by 70%. In the NCC inhibition simulations, baseline interstitial concentration profiles were used.
The predicted fractional Mg2+ excretion after NCC inhibition increased to 3.1% from the baseline value of 2.8% (Figure 3). This is in agreement with experimental data where male rats treated with bendrofluazide, a thiazide diuretic, did not show any significant change in Mg2+ excretion [35]. Acute administration of chlorothiazide to male mice increased TRPV5 mRNA expression by 40–80% and decreased Ca2+ excretion by ~60% [36]. Accordingly, we increased TRPV5 activity by 52% following NCC inhibition in our model. This decreased the predicted Ca2+ excretion by 63% from the baseline excretion value (Figure 3).
3.4. K-Sparing Diuretics
K-sparing diuretics, such as amiloride, block Na+ uptake through ENaC, expressed on the apical membrane of the late distal convoluted tubule as well as along the full length of the connecting tubule and collecting ducts. In our model, we simulated the effect of K-sparing diuretics by reducing ENaC activity by 70%.
ENaC inhibition hyperpolarizes the luminal membrane potential and increases K+, Ca2+, and Mg2+ uptake [37,38,39,40]. Our model simulations predicted Mg2+ reabsorption along the distal convoluted tubule to increase by 8.2% and Ca2+ reabsorption along the distal convoluted tubule and connecting tubule to increase by 29% (Figure 3). These increased reabsorptions decreased Mg2+ and Ca2+ excretions by 31% and 56%, respectively (Figure 3).
3.5. TRPM6/7 Inhibition
Kidney-specific TRPM6 [41] and TRPM7 [42] knock-out mice did not display hypomagnesemia and increased urinary Mg2+ excretion. This indicates that in mice, there must be other Mg2+ uptake mechanisms in the distal convoluted tubule. In fact, Verschuren et al. [43] reported that fluid shear stress (FSS) stimulated Mg2+ uptake in mDCT15 cells, and this uptake was independent of the TRPM6 and TRPM7 channels. However, the pathways or regulatory mechanisms for this FSS-sensitive Mg2+ uptake are unclear and hence not included in our present model.
To simulate TRPM6/7 knock-out experiments, we inhibited the TRPM6/7 channel by 100%. Our model predicted fractional Mg2+ excretion to increase to 8.8% (from baseline 3.2%), which is significantly above the 2–5% physiological fractional Mg2+ excretion. How much should the Mg2+ uptake through the FSS-sensitive Mg2+ channel be for fractional Mg2+ excretion to be within the physiological range when TRPM6/7 is completely inhibited? Our simulations predicted that if Mg2+ uptake through the FSS-sensitive channel is at least 60% of the Mg2+ uptake through TRPM6/7, then the fractional Mg2+ excretion becomes 4.3% (within the physiological range).
4. Discussion
Calcium (Ca2+) and magnesium (Mg2+) are both essential for cellular function. The homeostasis of these cations must be tightly regulated, and that balance is facilitated by intestinal absorption and renal excretion. For Na+, Cl−, K+, Ca2+, and many other major filtered solutes, most of the renal reabsorption occurs along the proximal convoluted tubule (about 1/2 to 2/3 in rats); the same is true for water. Thus, the luminal concentrations of these solutes, including Ca2+, remain close to plasma along the proximal convoluted tubule. The majority of proximal tubule Ca2+ reabsorption occurs via a passive paracellular process, driven by Na+ reabsorption mediated primarily by NHE3 and subsequent water reabsorption. In contrast, only 15–25% of the filtered Mg2+ load is reabsorbed along the proximal tubule. As a result, its concentration rises significantly along the proximal tubule. For Mg2+, most of the reabsorption occurs along the cortical thick ascending limb (about 60–70%), while somewhat unexpectedly, essentially none occurs along the medullary thick ascending limb. Most of the remainder of the Mg2+ is reabsorbed along the distal convoluted tubule.
What difference does it make for the cortical thick ascending limb and distal convoluted tubule to handle most of the Mg2+ transport instead of the proximal tubule, as in the case of Na+ and Cl−? Having these distal segments responsible for transporting a substantial fraction of the filtered Mg2+ load via the pathways that can be regulated may give the kidney a better ability to regulate Mg2+ balance. Recall that the plasma Mg2+ level is orders of magnitude lower than Na+ or Cl−. Thus, to maintain plasma [Mg2+] within a narrow range, the ability to fine-tune renal Mg2+ transport is particularly crucial. Parathyroid hormone, for instance, increases Mg2+ reabsorption in both the cortical thick ascending limb and distal convoluted tubule [44]. Transport of Mg2+ along these segments can also be regulated by hormones such as calcitonin, vasopressin, glucagon, and β-adrenergic agonists [45]. Coincidentally, some common diuretics also target these segments.
The goal of this study is to better understand the impact of diuretics on renal Ca2+ and Mg2+ transport. Diuretics are medications that reduce fluid buildup in the body and are often employed in the management of hypertension, edema, and various other conditions influenced by changes in electrolyte transport. In the context of kidney function, diuretics often focus on transport proteins or mechanisms vital for the reabsorption of Na+, Cl−, and water. Considering that renal Ca2+ and Mg2+ transports are driven primarily by the electrochemical gradients established by tubular NaCl transport processes, potential modifications in the renal handling of Ca2+ and Mg2+ by the administration of diuretics deserve scrutiny.
Loop diuretics, such as furosemide, induce notable natriuresis by targeting the thick ascending limb of the nephron; specifically, they inhibit NKCC2-dependent transport by competing for the chloride (Cl−) binding site [46]. Given that the inhibition of NKCC2 reduces the lumen-positive transepithelial voltage gradient across the thick ascending limb epithelium [47], it is unsurprising that the transport of Mg2+ and Ca2+ in this segment is substantially decreased [48,49]. In fact, loop diuretics like furosemide induce hypercalciuria and hypermagnesuria in both experimental animals [50] and human subjects [51]. Thiazide diuretics induce a natriuretic response by inhibiting NCC and blocking NaCl transport in the distal convoluted tubule. A hypocalciuric effect has been reported following thiazide treatment [52]. Consistent with the drug’s mode of action, among hypertensive individuals undergoing chronic thiazide treatment, there is a slight decrease in serum Mg2+ levels compared to those not taking diuretics [53], although the effect appears to be subtle. K-sparing diuretics function by inhibiting ENaC, a protein expressed in the late distal convoluted tubule, connecting tubule, and collecting duct of the kidney. Research has confirmed that K-sparing diuretics impact the urinary excretion of Ca2+ and Mg2+ in both human subjects and animals. Hypertensive individuals undergoing K-sparing diuretics treatment exhibit reduced urinary Ca2+ excretion [53] and elevated serum Mg2+ levels compared to those not receiving treatment [53]. The effect of these three classes of diuretics on urinary Mg2+ and Ca2+ excretions has been summarized in Figure 4.
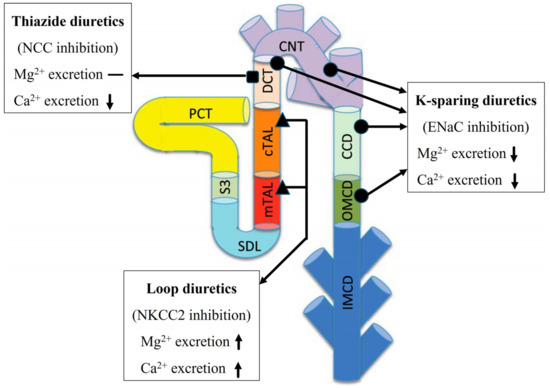
Figure 4.
Summary of the effects of diuretic treatment. The effect of loop diuretics, thiazide diuretics, and K-sparing diuretics on Mg2+ and Ca2+ excretion The upward arrow indicates an increase, the downward arrow indicates a decrease, and the dash indicates no significant change. Notations are analogous to Figure 1.
The present study considers renal Ca2+ and Mg2+ transport under normal physiological conditions. The homeostasis of these electrolytes is altered during pregnancy, lactation, and dietary restriction, as well as in diseases such as diabetes and chronic kidney disease. To utilize the present model for in silico studies of how the kidney adapts in terms of Ca2+ and Mg2+ transport under these physiological and pathophysiological conditions, one can combine the model with computational models of kidney function for a pregnant rat [54,55], a diabetic rat [56,57,58], and a nephrectomized rat [8,59]. The resulting models may provide insights into altered renal Ca2+ and Mg2+ transport under these conditions. How do changes in renal Ca2+ and Mg2+ transport impact whole-body Ca2+ and Mg2+ homeostasis? A whole-body Ca2+ and Mg2+ balance model may help answer that question. By incorporating the present model into whole-body electrolyte balance models (e.g., [60,61,62,63]), one can obtain an integrative model to study whole-body Ca2+ and Mg2+ balance.
The present model simulates electrolyte and water transport along a superficial nephron, which constitutes only 2/3 of the total nephron population in a rat kidney. The juxtamedullary nephrons, whose loops of Henle extend into various depths of the inner medulla, comprise the remaining nephron population. These two types of nephrons differ in the single-nephron glomerular filtration rate, transport area, and transporter activities. This study focuses on a superficial nephron model to gain a clearer understanding of segmental Mg2+ transport. In future studies, developing a kidney model that incorporates both types of nephrons will enable more accurate predictions of urinary excretion rates.
Another limitation of this model is that it does not include cyclin and CBS domain divalent metal cation transport mediator 2 (CNNM2), expressed on the basolateral membrane, which is also potentially involved in Mg2+ transport along the distal convoluted tubule. CNNM2 has been shown to mediate both cellular Mg2+ influx and efflux. The role of CNNM2 as an Mg2+ transporter has been openly debated (in favor [64]; opposing view [65]). A common view is that CNNM2 is not a Mg2+ transporter by itself but is an important protein for regulating Mg2+ homeostasis [65,66]. Due to these conflicting views on the role of CNNM2 as a Mg2+ transporter, we did not include it in our present model. CNNM2 can be incorporated into our model in the future when more consistent experimental outcomes are available.
Author Contributions
Conceptualization, A.T.L.; Methodology, P.D. and A.T.L.; Software, Validation, Formal Analysis, and Investigation, P.D.; Resources, A.T.L.; Data Curation, P.D.; Writing—Original Draft, P.D. and A.T.L.; Writing—Review and Editing, P.D. and A.T.L.; Visualization, P.D.; Supervision, P.D. and A.T.L.; Funding Acquisition, A.T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Canada 150 Research Chair program, the National Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (Grant number: RGPIN-2019-03916), and the Canada Institutes of Health Research (CIHR) Project Grant (Grant number: TNC-174963) (to A.T.L.).
Data Availability Statement
The code used for this study can be accessed at https://github.com/Pritha17/Nephron-Mg_Ca_transport (16 October 2023).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pu, F.; Chen, N.; Xue, S. Calcium intake, calcium homeostasis and health. Food Sci. Hum. Wellness 2016, 5, 8–16. [Google Scholar] [CrossRef]
- Taal, M.W.; Chertow, G.M.; Marsden, P.A.; Skorecki, K.; Alan, S.; Brenner, B.M. Brenner and Rector’s the Kidney E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011; ISBN 1-4557-2304-5. [Google Scholar]
- Alexander, R.T.; Dimke, H. Effect of diuretics on renal tubular transport of calcium and magnesium. Am. J. Physiol. Ren. Physiol. 2017, 312, F998–F1015. [Google Scholar] [CrossRef]
- Brunette, M.; Vigneault, N.; Carriere, S. Micropuncture study of magnesium transport along the nephron in the young rat. Am. J. Physiol. Leg. Content 1974, 227, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Le Grimellec, C.; Giocondi, M.C.; Philippe, P. Micropuncture study along the proximal convoluted tubule electrolyte reabsorption in first convolutions. Pflug. Arch. 1975, 354, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.N.; Yu, A.S.L. Magnesium Handling in the Kidney. Adv. Chronic Kidney Dis. 2018, 25, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.T.; Layton, H.E. A Computational Model of Epithelial Solute and Water Transport along a Human Nephron. PLoS Comput. Biol. 2018, 15, e1006108. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.T.; Vallon, V. SGLT2 Inhibition in a Kidney with Reduced Nephron Number: Modeling and Analysis of Solute Transport and Metabolism. Am. J. Physiol. Ren. Physiol. 2018, 314, F969–F984. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.T.; Laghmani, K.; Vallon, V.; Edwards, A. Solute transport and oxygen consumption along the nephrons: Effects of Na+ transport inhibitors. Am. J. Physiol. Ren. Physiol. 2016, 311, F1217–F1229. [Google Scholar] [CrossRef]
- Hakimi, S.; Dutta, P.; Layton, A.T. Coupling of renal sodium and calcium transport: A modeling analysis of transporter inhibition and sex differences. Am. J. Physiol. Ren. Physiol. 2023, 325, F536–F551. [Google Scholar] [CrossRef]
- Layton, A. A Complete Set of Equations for a Computational Model of Electrolyte and Water Transport along the Nephrons in a Mammalian Kidney. bioRxiv 2022, bioRxiv:509286. [Google Scholar] [CrossRef]
- Layton, A.T.; Vallon, V.; Edwards, A. A computational model for simulating solute transport and oxygen consumption along the nephrons. Am. J. Physiol. Ren. Physiol. 2016, 311, F1378–F1390. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.T.; Dimke, H. Molecular mechanisms underlying paracellular calcium and magnesium reabsorption in the proximal tubule and thick ascending limb. Ann. N. Y. Acad. Sci. 2022, 1518, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Topham, D.J.; Park, S.Y.; Hollenbaugh, J.; Treanor, J.; Mosmann, T.R.; Jin, X.; Ward, B.M.; Miao, H.; Holden-Wiltse, J. Simulation and prediction of the adaptive immune response to influenza A virus infection. J. Virol. 2009, 83, 7151–7165. [Google Scholar] [CrossRef] [PubMed]
- Wittner, M.; di Stefano, A.; Wangermann, P.; Nitschke, R.; Greger, R.; Bailly, C.; Amiel, C.; Roinel, N.; de Rouffignac, C. Differential effects of ADH on sodium, chloride, potassium, calcium and magnesium transport in cortical and medullary thick ascending limbs of mouse nephron. Pflug. Arch. 1988, 412, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Quamme, G.A. Control of magnesium transport in the thick ascending limb. Am. J. Physiol. Ren. Physiol. 1989, 256, F197–F210. [Google Scholar] [CrossRef]
- Di Stefano, A.; Wittner, M.; Nitschke, R.; Braitsch, R.; Greger, R.; Bailly, C.; Amiel, C.; Roinel, N.; de Rouffignac, C. Effects of parathyroid hormone and calcitonin on Na+, Cl−, K+, Mg2+ and Ca2+ transport in cortical and medullary thick ascending limbs of mouse kidney. Pflug. Arch. 1990, 417, 161–167. [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Franken, G.A.C.; Adella, A.; Bindels, R.J.M.; de Baaij, J.H.F. Mechanisms coupling sodium and magnesium reabsorption in the distal convoluted tubule of the kidney. Acta Physiol. 2021, 231, e13528. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.; Yue, L. Functional Characterization of Homo- and Heteromeric Channel Kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006, 127, 525–537. [Google Scholar] [CrossRef]
- Günther, T.; Vormann, J.; Höllriegl, V. Characterization of Na+-dependent Mg2+ efflux from Mg2+-loaded rat erythrocytes. Biochim. Biophys. Acta BBA Biomembr. 1990, 1023, 455–461. [Google Scholar] [CrossRef]
- Ruat, M.; Snowman, A.M.; Hester, L.D.; Snyder, S.H. Cloned and Expressed Rat Ca2+-sensing Receptor: Differential Cooperative Responses to Calcium and Magnesium. J. Biol. Chem. 1996, 271, 5972–5975. [Google Scholar] [CrossRef] [PubMed]
- Bräuner-Osborne, H.; Jensen, A.A.; Sheppard, P.O.; O’Hara, P.; Krogsgaard-Larsen, P. The Agonist-Binding Domain of the Calcium-Sensing Receptor Is Located at the Amino-terminal Domain. J. Biol. Chem. 1999, 274, 18382–18386. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Chen, C.J. Calcium, magnesium and the control of PTH secretion. Bone Miner. 1989, 5, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Valenti, G. Localization and function of the renal calcium-sensing receptor. Nat. Rev. Nephrol. 2016, 12, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Murayama, Y.; Morel, F.; Le Grimellec, C. Phosphate, calcium and magnesium transfers in proximal tubules and loops of henle, as measured by single nephron microperfusion experiments in the rat. Pflug. Arch. 1972, 333, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A. Regulation of calcium reabsorption along the rat nephron: A modeling study. Am. J. Physiol. Ren. Physiol. 2015, 308, F553–F566. [Google Scholar] [CrossRef]
- Toka, H.R.; Al-Romaih, K.; Koshy, J.M.; DiBartolo, S.I.; Kos, C.H.; Quinn, S.J.; Curhan, G.C.; Mount, D.B.; Brown, E.M.; Pollak, M.R. Deficiency of the Calcium-Sensing Receptor in the Kidney Causes Parathyroid Hormone–Independent Hypocalciuria. J. Am. Soc. Nephrol. 2012, 23, 1879. [Google Scholar] [CrossRef]
- Wang, W.H.; Lu, M.; Hebert, S.C. Cytochrome P-450 metabolites mediate extracellular Ca2+-induced inhibition of apical K+ channels in the TAL. Am. J. Physiol. Cell Physiol. 1996, 271, C103–C111. [Google Scholar] [CrossRef]
- Wang, W.; Lu, M.; Balazy, M.; Hebert, S.C. Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. Am. J. Physiol. Ren. Physiol. 1997, 273, F421–F429. [Google Scholar] [CrossRef]
- Bazúa-Valenti, S.; Rojas-Vega, L.; Castañeda-Bueno, M.; Barrera-Chimal, J.; Bautista, R.; Cervantes-Pérez, L.G.; Vázquez, N.; Plata, C.; Murillo-de-Ozores, A.R.; González-Mariscal, L.; et al. The Calcium-Sensing Receptor Increases Activity of the Renal NCC through the WNK4-SPAK Pathway. J. Am. Soc. Nephrol. 2018, 29, 1838. [Google Scholar] [CrossRef]
- Hu, R.; McDonough, A.A.; Layton, A.T. Functional implications of the sex differences in transporter abundance along the rat nephron: Modeling and analysis. Am. J. Physiol. Ren. Physiol. 2019, 317, F1462–F1474. [Google Scholar] [CrossRef] [PubMed]
- Van Angelen, A.A.; van der Kemp, A.W.; Hoenderop, J.G.; Bindels, R.J. Increased expression of renal TRPM6 compensates for Mg2+ wasting during furosemide treatment. Nephrol. Dial. Transplant. Plus 2012, 5, 535–544. [Google Scholar] [CrossRef]
- Devane, J.; Ryan, M. Diuretics and magnesium excretion. Magnes. Bull. 1981, 3, 122–123. [Google Scholar]
- Ryan, M.P.; Devane, J.; Ryan, M.F.; Counihan, T.B. Effects of diuretics on the renal handling of magnesium. Drugs 1984, 28, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Shang, S.; Lai, L.-W.; Yong, K.-C.; Lien, Y.-H.H. Effect of thiazide on renal gene expression of apical calcium channels and calbindins. Am. J. Physiol. Ren. Physiol. 2004, 287, F1164–F1170. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, L.S. Comparison of calcium and sodium transport in early and late rat distal tubules: Effect of amiloride. Am. J. Physiol. Ren. Physiol. 1984, 246, F937–F945. [Google Scholar] [CrossRef]
- Devane, J.; Ryan, M.P. The effects of amiloride and triamterene on urinary magnesium excretion in conscious saline-loaded rats. Br. J. Pharmacol. 1981, 72, 285. [Google Scholar] [CrossRef]
- Devane, J.; Ryan, M.P. Dose-dependent reduction in renal magnesium clearance by amiloride during frusemide-induced diuresis in rats. Br. J. Pharmacol. 1983, 80, 421. [Google Scholar] [CrossRef]
- Friedman, P.A.; Gesek, F.A. Stimulation of calcium transport by amiloride in mouse distal convoluted tubule cells. Kidney Int. 1995, 48, 1427–1434. [Google Scholar] [CrossRef]
- Chubanov, V.; Ferioli, S.; Wisnowsky, A.; Simmons, D.G.; Leitzinger, C.; Einer, C.; Jonas, W.; Shymkiv, Y.; Bartsch, H.; Braun, A.; et al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. eLife 2016, 5, e20914. [Google Scholar] [CrossRef]
- Mittermeier, L.; Demirkhanyan, L.; Stadlbauer, B.; Breit, A.; Recordati, C.; Hilgendorff, A.; Matsushita, M.; Braun, A.; Simmons, D.G.; Zakharian, E.; et al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl. Acad. Sci. USA 2019, 116, 4706–4715. [Google Scholar] [CrossRef]
- Verschuren, E.H.; Hoenderop, J.G.; Peters, D.J.; Arjona, F.J.; Bindels, R.J. Tubular flow activates magnesium transport in the distal convoluted tubule. FASEB J. 2019, 33, 5034–5044. [Google Scholar] [CrossRef]
- Bailly, C.; Roinel, N.; Amiel, C. PTH-like glucagon stimulation of Ca and Mg reabsorption in Henle’s loop of the rat. Am. J. Physiol. Ren. Physiol. 1984, 246, F205–F212. [Google Scholar] [CrossRef]
- Quamme, G.A.; de Rouffignac, C. Renal magnesium handling. In The Kidney: Physiology and Pathophysiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; p. 375. [Google Scholar]
- O’Grady, S.M.; Palfrey, H.C.; Field, M. Characteristics and functions of Na-K-Cl cotransport in epithelial tissues. Am. J. Physiol. Cell Physiol. 1987, 253, C177–C192. [Google Scholar] [CrossRef]
- Burg, M.B. Tubular chloride transport and the mode of action of some diuretics. Kidney Int. 1976, 9, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Roinel, N.; de Rouffignac, C.; Wittner, M. Transepithelial Ca2+ and Mg2+ Transport in the Cortical Thick Ascending Limb of Henle’s Loop of the Mouse Is a Voltage-Dependent Process. Ren. Physiol. Biochem. 2008, 16, 157–166. [Google Scholar] [CrossRef]
- Quamme, G.A. Effect of furosemide on calcium and magnesium transport in the rat nephron. Am. J. Physiol. Ren. Physiol. 1981, 241, F340–F347. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.G. Effects of ethacrynic acid and furosemide on urinary calcium, phosphate and magnesium. Metabolism 1968, 17, 867–876. [Google Scholar] [CrossRef]
- Leary, W.P.; Reyes, A.J.; Wynne, R.D.; van der Byl, K. Renal Excretory Actions of Furosemide, of Hydrochlorothiazide and of the Vasodilator Flosequinan in Healthy Subjects. J. Int. Med. Res. 1990, 18, 120–141. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.S.; Massry, S.G.; Coburn, J.W. Changes in Serum and Urinary Calcium during Treatment with Hydrochlorothiazide: Studies on Mechanisms. J. Clin. Investig. 1972, 51, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Wood, D.R.; Hanley, J.F. The Value of Serum Magnesium Determination in Hypertensive Patients Receiving Diuretics. Arch. Intern. Med. 1987, 147, 1553–1556. [Google Scholar] [CrossRef]
- Stadt, M.M.; Layton, A.T. Adaptive changes in single-nephron GFR, tubular morphology, and transport in a pregnant rat nephron: Modeling and analysis. Am. J. Physiol. Ren. Physiol. 2022, 322, F121–F137. [Google Scholar] [CrossRef]
- Stadt, M.M.; West, C.A.; Layton, A.T. Effect of pregnancy and hypertension on kidney function in female rats: Modeling and functional implications. PLoS ONE 2023, 18, e0279785. [Google Scholar] [CrossRef]
- Layton, A.T.; Vallon, V.; Edwards, A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am. J. Physiol. Ren. Physiol. 2016, 310, F1269–F1283. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Layton, A. A computational model of kidney function in a patient with diabetes. Int. J. Mol. Sci. 2021, 22, 5819. [Google Scholar] [CrossRef]
- Swapnasrita, S.; Carlier, A.; Layton, A.T. Sex-specific computational models of kidney function in patients with diabetes. Front. Physiol. 2022, 13, 741121. [Google Scholar] [CrossRef]
- Layton, A.T.; Edwards, A.; Vallon, V. Renal potassium handling in rats with subtotal nephrectomy: Modeling and Analysis. Am. J. Physiol. Ren. Physiol. 2017, 314, F643–F657. [Google Scholar] [CrossRef]
- Stadt, M.M.; Layton, A.T. Mathematical modeling of calcium homeostasis in female rats: An analysis of sex differences and maternal adaptations. J. Theor. Biol. 2023, 572, 111583. [Google Scholar] [CrossRef] [PubMed]
- Stadt, M.M.; Leete, J.; Devinyak, S.; Layton, A.T. A mathematical model of potassium homeostasis: Effect of feedforward and feedback controls. PLoS Comput. Biol. 2022, 18, e1010607. [Google Scholar] [CrossRef] [PubMed]
- Leete, J.; Layton, A.T. Sex-specific long-term blood pressure regulation: Modeling and analysis. Comput. Biol. Med. 2019, 104, 139–148. [Google Scholar] [CrossRef]
- Ahmed, S.; Layton, A.T. Sex-specific computational models for blood pressure regulation in the rat. Am. J. Physiol. Ren. Physiol. 2020, 318, F888–F900. [Google Scholar] [CrossRef] [PubMed]
- Funato, Y.; Furutani, K.; Kurachi, Y.; Miki, H. CrossTalk proposal: CNNM proteins are Na+/Mg2+ exchangers playing a central role in transepithelial Mg2+ (re)absorption. J. Physiol. 2018, 596, 743. [Google Scholar] [CrossRef] [PubMed]
- Arjona, F.J.; de Baaij, J.H. CrossTalk opposing view: CNNM proteins are not Na+/Mg2+ exchangers but Mg2+ transport regulators playing a central role in transepithelial Mg2+ (re)absorption. J. Physiol. 2018, 596, 747. [Google Scholar] [CrossRef] [PubMed]
- Sponder, G.; Mastrototaro, L.; Kurth, K.; Merolle, L.; Zhang, Z.; Abdulhanan, N.; Smorodchenko, A.; Wolf, K.; Fleig, A.; Penner, R.; et al. Human CNNM2 is not a Mg2+ transporter per se. Pflug. Arch. Eur. J. Physiol. 2016, 468, 1223–1240. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).