Aggressive Cutaneous Squamous Cell Carcinomas Following Treatment for Graft-versus-Host Disease: A Case Report and Review of Risk Factors
Abstract
1. Introduction
- Distinction from acute GVHD;
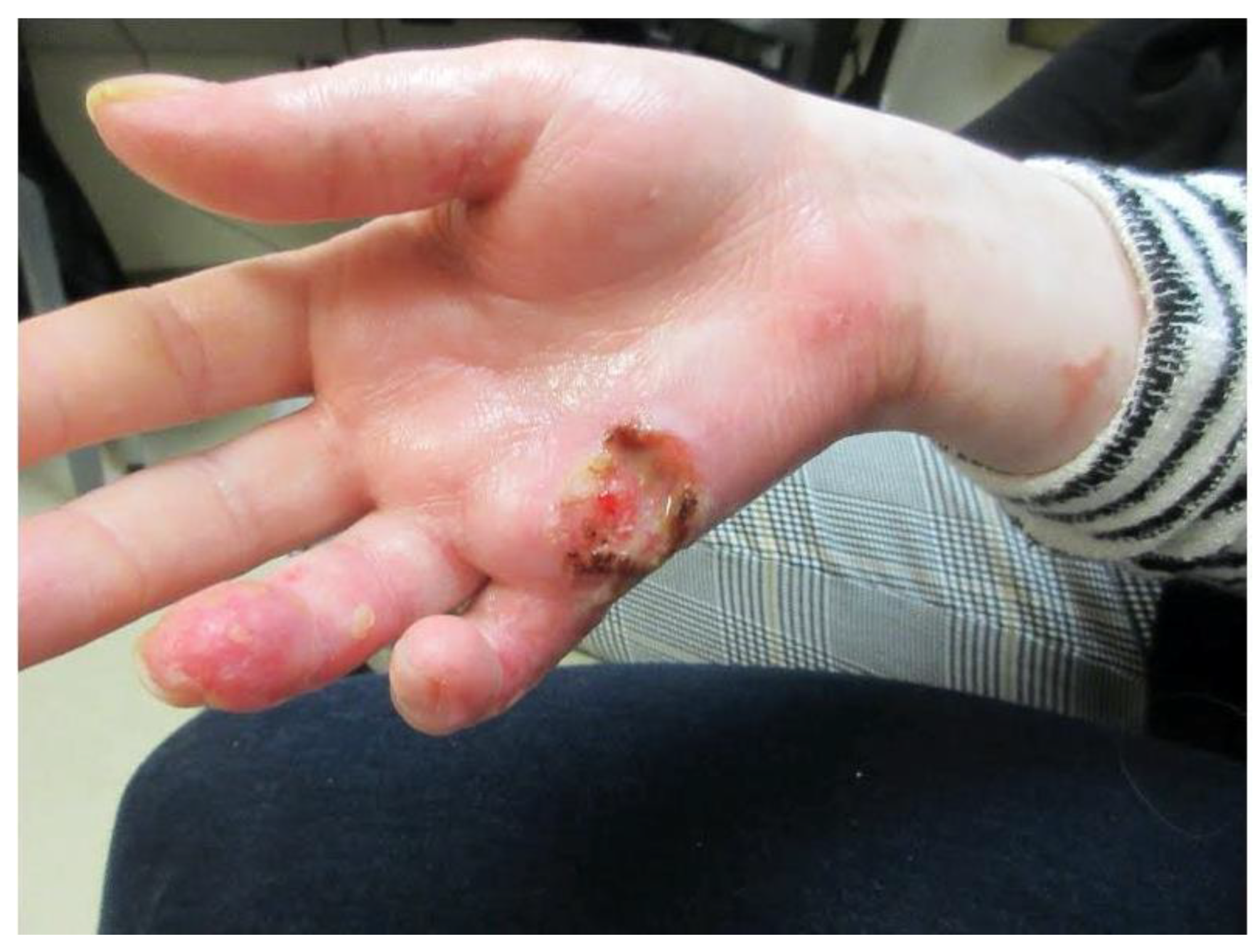
- Presence of at least one diagnostic clinical sign of chronic GVHD or presence of at least one distinctive manifestation confirmed by biopsy or other relevant tests (Figure 1);
- Exclusion of other possible diagnoses.
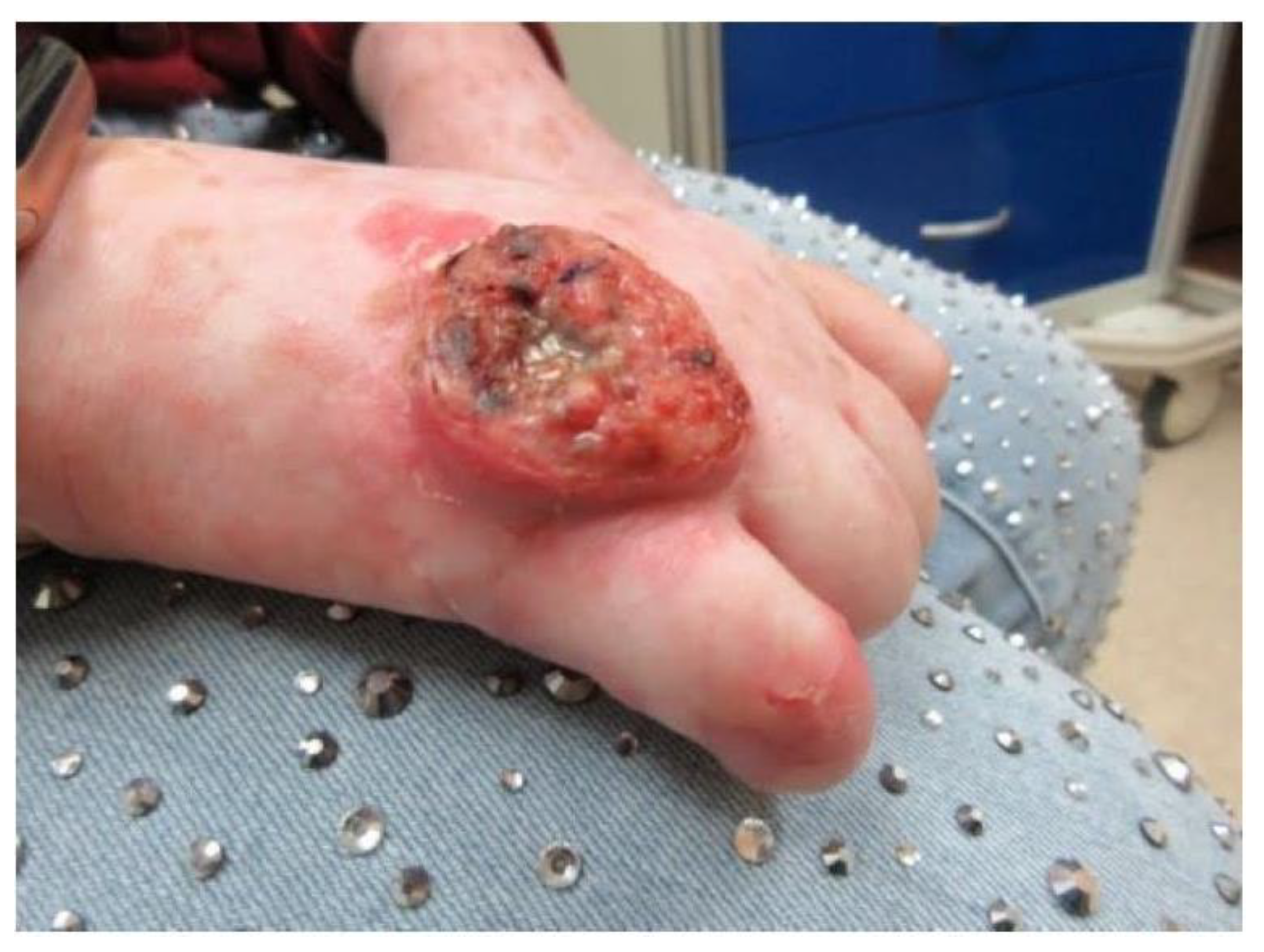
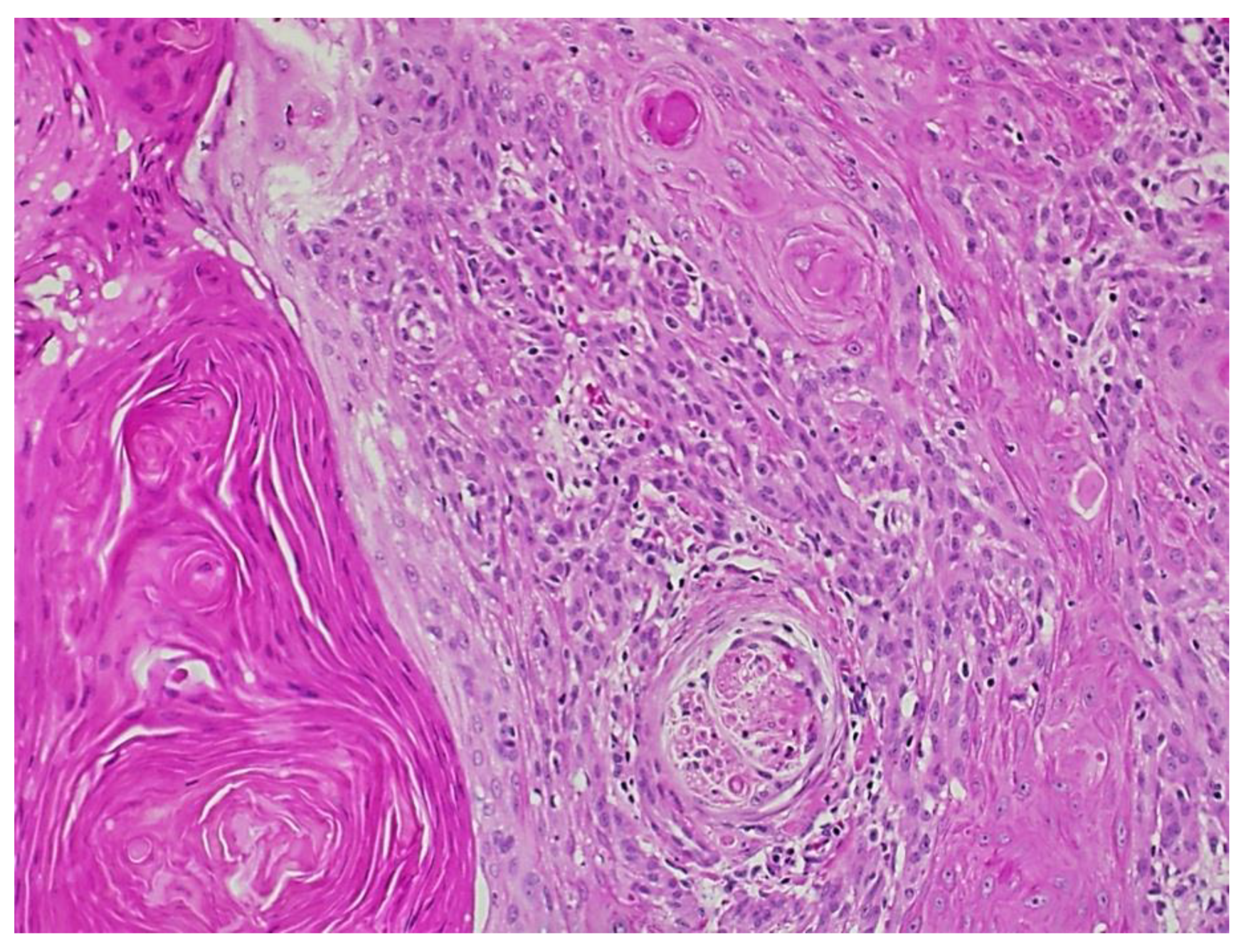
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curtis, R.E.; Rowlings, P.A.; Deeg, H.J.; Shriner, D.A.; Socié, G.; Travis, L.B.; Horowitz, M.M.; Whiterspoon, R.P.; Hoover, R.N.; Sobocinski, K.A.; et al. Solid cancers after bone marrow transplantation. N. Engl. J. Med. 1997, 336, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.A.; Toland, A.E.; Proby, C.M.; Euvrard, S.; Hofbauer, G.F.L.; Tommasino, M.; Bouwes Bavinck, J.N. The pathogenesis of cutaneous squamous cell carcinoma in organ transplant recipients. Br. J. Dermatol. 2017, 177, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Leisenring, W.; Friedman, D.L.; Flowers, M.E.D.; Schwartz, J.L.; Deeg, H.J. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J. Clin. Oncol. 2006, 24, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Fortina, A.B.; Piaserico, S.; Caforio, A.L.P.; Albeni, D.; Alaibac, M.; Angelini, A.; Iliceto, S.; Peserico, A. Immunosuppressive level and other risk factors for basal cell carcinoma and squamous cell carcinoma in heart transplant recipients. Arch. Derm. 2004, 140, 1079–1485. [Google Scholar] [CrossRef] [PubMed]
- DePry, J.L.; Vyas, R.; Lazarus, H.M.; Caimi, P.F.; Gerstenblith, M.R.; Bordeaux, J.S. Cutaneous Malignant Neoplasms in Hematopoietic Cell Transplant Recipients: A Systematic Review. JAMA Derm. 2015, 151, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.A.; Stern, R.S.; Huang, V.; Liu, K.X.; Chen, A.; Tzachanis, D.; Joyce, R.M.; Davis, R.B.; Ho, V.T. Reduced-Intensity Conditioning Regimens, Prior Chronic Lymphocytic Leukemia, and Graft-Versus-Host Disease Are Associated with Higher Rates of Skin Cancer after Allogeneic Hematopoietic Stem Cell Transplantation. J. Investig. Derm. 2019, 139, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.A.; Modi, P.; Mohammadi, O. Graft Versus Host Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lee, S.J.; Vogelsang, G.; Flowers, M.E. Chronic graft-versus-host disease. Biol. Blood Marrow Transpl. 2003, 9, 215–233. [Google Scholar] [CrossRef]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transpl. 2005, 11, 945–956. [Google Scholar] [CrossRef]
- Jagasia, M.; Giglia, J.; Chinratanalab, W.; Dixon, S.; Chen, H.; Frangoul, H.; Engelhardt, B.; Goodman, S.; Greer, J.; Kassim, A.; et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol. Blood Marrow Transpl. 2007, 13, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Hymes, S.R.; Turner, M.L.; Champlin, R.E.; Couriel, D.R. Cutaneous manifestations of chronic graft-versus-host disease. Biol. Blood Marrow Transpl. 2006, 12, 1101–1113. [Google Scholar] [CrossRef][Green Version]
- Rangwala, S.; Tsai, K.Y. Roles of the immune system in skin cancer. Br. J. Dermatol. 2011, 165, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Kohler, S.; Hendrickson, M.R.; Chao, N.J.; Smoller, B.R. Value of skin biopsies in assessing prognosis and progression of acute graft-versus-host disease. Am. J. Surg. Pathol. 1997, 21, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Sale, G.E.; Lerner, K.G.; Barker, E.A.; Shulman, H.M.; Thomas, E.D. The skin biopsy in the diagnosis of acute graft-versus-host disease in man. Am. J. Pathol. 1977, 89, 621–636. [Google Scholar] [PubMed]
- Rambhia, P.H.; Conic, R.Z.; Atanaskova-Mesinkovska, N.; Piliang, M.; Bergfeld, W.F. Role of graft-versus-host disease in the development of secondary skin cancers in hematopoietic stem cell transplant recipients: A meta-analysis. J. Am. Acad. Dermatol. 2018, 79, 378–380.e3. [Google Scholar] [CrossRef] [PubMed]
- Aldabagh, B.; Angeles, J.G.; Cardones, A.R.; Arron, S.T. Cutaneous squamous cell carcinoma and human papillomavirus: Is there an association? Dermatol. Surg. 2013, 39, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Koehler, A.; Forschner, T.; Sehr, P.; Michael, K.; Pawlita, M.; Stockfleth, E.; Nindl, I. E6/E7 expression of human papillomavirus types in cutaneous squamous cell dysplasia and carcinoma in immunosuppressed organ transplant recipients. Br. J. Dermatol. 2006, 155, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Iaconis, L.; Hyjek, E.; Ellenson, L.H.; Pirog, E.C. p16 and Ki-67 immunostaining in atypical immature squamous metaplasia of the uterine cervix: Correlation with human papillomavirus detection. Arch. Pathol. Lab. Med. 2007, 131, 1343–1349, Correction in Arch. Pathol. Lab. Med. 2008, 132, 13. [Google Scholar] [CrossRef]
- Adham, M.; Aldino, N.; Zahra, S.; Rachmadi, L.; Bardosono, S. Feasibility of p16 surrogate biomarker as adjunct diagnosis of oral and oropharyngeal malignancy in a resource-constrained country. Acta Otolaryngol. 2021, 141, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Doxtader, E.E.; Katzenstein, A.L. The relationship between p16 expression and high-risk human papillomavirus infection in squamous cell carcinomas from sites other than uterine cervix: A study of 137 cases. Hum. Pathol. 2012, 43, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Caparrotti, F.; Troussier, I.; Ali, A.; Zilli, T. Localized Non-melanoma Skin Cancer: Risk Factors of Post-surgical Relapse and Role of Postoperative Radiotherapy. Curr. Treat. Options Oncol. 2020, 21, 97. [Google Scholar] [CrossRef]
- Gertler, R.; Werber, K.D. Management of verrucous carcinoma of the hand: A case report. Int. J. Derm. 2009, 48, 1233–1235. [Google Scholar] [PubMed]
- Nehal, K.S.; Bichakjian, C.K. Update on Keratinocyte Carcinomas. N. Engl. J. Med. 2018, 379, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, P.; Asgari, M.M.; Green, A.C.; Guhan, S.M.; Arron, S.T.; Proby, C.M.; Rollison, D.E.; Harwood, C.A.; Toland, A.E. Keratinocyte Carcinomas: Current Concepts and Future Research Priorities. Clin. Cancer Res. 2019, 25, 2379–2391. [Google Scholar] [CrossRef]
- Omland, S.H.; Gniadecki, R.; Hædersdal, M.; Helweg-Larsen, J.; Omland, L.H. Skin Cancer Risk in Hematopoietic Stem-Cell Transplant Recipients Compared with Background Population and Renal Transplant Recipients: A Population-Based Cohort Study. JAMA Dermatol. 2016, 152, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kuklinski, L.F.; Li, S.; Karagas, M.R.; Weng, W.K.; Kwong, B.Y. Effect of voriconazole on risk of nonmelanoma skin cancer after hematopoietic cell transplantation. J. Am. Acad. Dermatol. 2017, 77, 706–712. [Google Scholar] [CrossRef]
- Williams, K.; Mansh, M.; Chin-Hong, P.; Singer, J.; Arron, S.T. Voriconazole-associated cutaneous malignancy: A literature review on photocarcinogenesis in organ transplant recipients. Clin. Infect. Dis. 2014, 58, 997–1002. [Google Scholar] [CrossRef]
- Inamoto, Y.; Shah, N.N.; Savani, B.N.; Shaw, B.E.; Abraham, A.A.; Ahmed, I.A.; Akpek, G.; Atsuta, Y.; Baker, K.S.; Basak, G.W.; et al. Secondary solid cancer screening following hematopoietic cell transplantation. Bone Marrow Transpl. 2015, 50, 1013–1023. [Google Scholar] [CrossRef]
- Rizzo, J.D.; Curtis, R.E.; Socié, G.; Sobocinski, A.K.; Gilbert, E.; Landgren, O.; Travis, L.B.; Flowers, M.E.D.; Friedman, D.L.; Horowitz, M.M.; et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood 2009, 113, 1175–1183. [Google Scholar] [CrossRef]
- Wolff, D.; Lawitschka, A. Chronic Graft-Versus-Host Disease. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Carreras, E., Dufour, C., Mohty, M., Kröger, N., Eds.; Springer: Cham, Switzerland, 2019; pp. 331–345. [Google Scholar]
- Horwitz, M.E.; Sullivan, K.M. Chronic graft-versus-host disease. Blood Rev. 2006, 20, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Kopecky, K.J.; Mathes, R.W.; Leisenring, W.M.; Friedman, D.L.; Deeg, H.J. Basal Cell Skin Cancer after Total-Body Irradiation and Hematopoietic Cell Transplantation. Rad. Res. 2009, 171, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, M.J.; Thomson, J.; Harwood, C.; Leigh, I. The Role of the Immune System in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2009. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R. Activation of Innate Immunity in Graft-versus-Host Disease: Implications for Novel Targets? Oncol. Res. Treat. 2015, 38, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, Y.; Bowlus, C.L.; Shimoda, S.; Ishibashi, H.; Vierling, J.M.; Gershwin, M.E. T cell immunity and graft-versus-host disease (GVHD). Autoimmun. Rev. 2006, 5, 1–9. [Google Scholar] [CrossRef]
- Plasmeijer, E.I.; Sachse, M.M.; Gebhardt, C.; Geusau, A.; Bouwes Bavinck, J.N. Cutaneous squamous cell carcinoma (cSCC) and immunosurveillance—The impact of immunosuppression on frequency of cSCC. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 33–37. [Google Scholar] [CrossRef]
- Coghill, A.E.; Johnson, L.G.; Berg, D.; Resler, A.J.; Leca, N.; Madeleine, M.M. Immunosuppressive Medications and Squamous Cell Skin Carcinoma: Nested Case-Control Study Within the Skin Cancer after Organ Transplant (SCOT) Cohort. Am. J. Transpl. 2016, 16, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Puckett, Y.; Gabbar, A.; Bokhari, A.A. Prednisone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Baibergenova, A.T.; Weinstock, M.A.; VATTC Trial Group. Oral prednisone use and risk of keratinocyte carcinoma in non-transplant population. The VATTC trial. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1109–1115. [Google Scholar] [CrossRef]
- Brazzelli, V.; Grasso, V.; Muzio, F.; Moggio, E.; Zecca, M.; Locatelli, F.; Borroni, G. Narrowband ultraviolet B phototherapy in the treatment of cutaneous graft-versus-host disease in oncohaematological paediatric patients. Br. J. Dermatol. 2010, 162, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, N.; Ozdemir Cetinkaya, P.; Kutlu, O.; Karaaslan, E.; Imren, I.G.; Nalbant, E.K.; Eksioglu, M. A cross-sectional analysis of skin cancer risk in patients receiving narrow-band ultraviolet B phototherapy: An evaluation of 100 patients. Arch. Dermatol. Res. 2020, 312, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Hearn, R.M.; Kerr, A.C.; Rahim, K.F.; Ferguson, J.; Dawe, R.S. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br. J. Dermatol. 2008, 159, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Wu, C.Y.; Chang, Y.T.; Juan, C.K.; Chen, C.C.; Yu, S.H.; Chen, Y.J. Risk of skin cancer in psoriasis patients receiving long-term narrowband ultraviolet phototherapy: Results from a Taiwanese population-based cohort study. Photodermatol. Photoimmunol. Photomed. 2019, 35, 164–171. [Google Scholar] [CrossRef]
- Stern, R.S.; PUVA Follow-Up Study. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: A 30-year prospective study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar] [CrossRef]
- Martin, T.; Sharma, M.; Damon, L.; Kaplan, L.; Guglielmo, B.J.; Working, M.; O’Malley, R.; Hwang, J.; Linker, C. Voriconazole is safe and effective as prophylaxis for early and late fungal infections following allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2010, 12, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.H.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef]
- Singer, J.P.; Boker, A.; Metchnikoff, C.; Binstock, M.; Boettger, R.; Golden, J.A.; Glidden, D.V.; Arron, S.T. High cumulative dose exposure to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J. Heart Lung Transpl. 2012, 31, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Mansh, M.; Binstock, M.; Williams, K.; Hafeez, F.; Kim, J.; Glidden, D.; Boettger, R.; Hays, S.; Kukreja, J.; Golden, J.; et al. Voriconazole Exposure and Risk of Cutaneous Squamous Cell Carcinoma, Aspergillus Colonization, Invasive Aspergillosis and Death in Lung Transplant Recipients. Am. J. Transpl. 2016, 16, 262–270. [Google Scholar] [CrossRef]
- Ona, K.; Oh, D.H. Voriconazole N-oxide and its ultraviolet B photoproduct sensitize keratinocytes to ultraviolet A. Br. J. Dermatol. 2015, 173, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Petra, B. Non-melanoma skin cancer: What drives tumor development and progression? Carcinogenesis 2005, 26, 1657–1667. [Google Scholar] [CrossRef]
- Euvrard, S.; Ulrich, C.; Lefrancois, N. Immunosuppressants and skin cancer in transplant patients: Focus on rapamycin. Dermatol. Surg. 2004, 30, 628–633. [Google Scholar] [CrossRef] [PubMed]


| Transplant-associated risk factors include: |
| Host-associated risk factors include: |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pendlebury, G.A.; Bongiorno, M.A.; Lackey, J.N. Aggressive Cutaneous Squamous Cell Carcinomas Following Treatment for Graft-versus-Host Disease: A Case Report and Review of Risk Factors. Dermatopathology 2022, 9, 122-130. https://doi.org/10.3390/dermatopathology9020015
Pendlebury GA, Bongiorno MA, Lackey JN. Aggressive Cutaneous Squamous Cell Carcinomas Following Treatment for Graft-versus-Host Disease: A Case Report and Review of Risk Factors. Dermatopathology. 2022; 9(2):122-130. https://doi.org/10.3390/dermatopathology9020015
Chicago/Turabian StylePendlebury, Gehan A., Michelle A. Bongiorno, and Jeffrey N. Lackey. 2022. "Aggressive Cutaneous Squamous Cell Carcinomas Following Treatment for Graft-versus-Host Disease: A Case Report and Review of Risk Factors" Dermatopathology 9, no. 2: 122-130. https://doi.org/10.3390/dermatopathology9020015
APA StylePendlebury, G. A., Bongiorno, M. A., & Lackey, J. N. (2022). Aggressive Cutaneous Squamous Cell Carcinomas Following Treatment for Graft-versus-Host Disease: A Case Report and Review of Risk Factors. Dermatopathology, 9(2), 122-130. https://doi.org/10.3390/dermatopathology9020015






