Histopathologic Features of Maculopapular Drug Eruption
Abstract
:1. Introduction
2. Pathophysiology
3. Risk Factors
4. Differential Diagnosis
5. Histopathologic Features of Maculopapular Drug Eruption
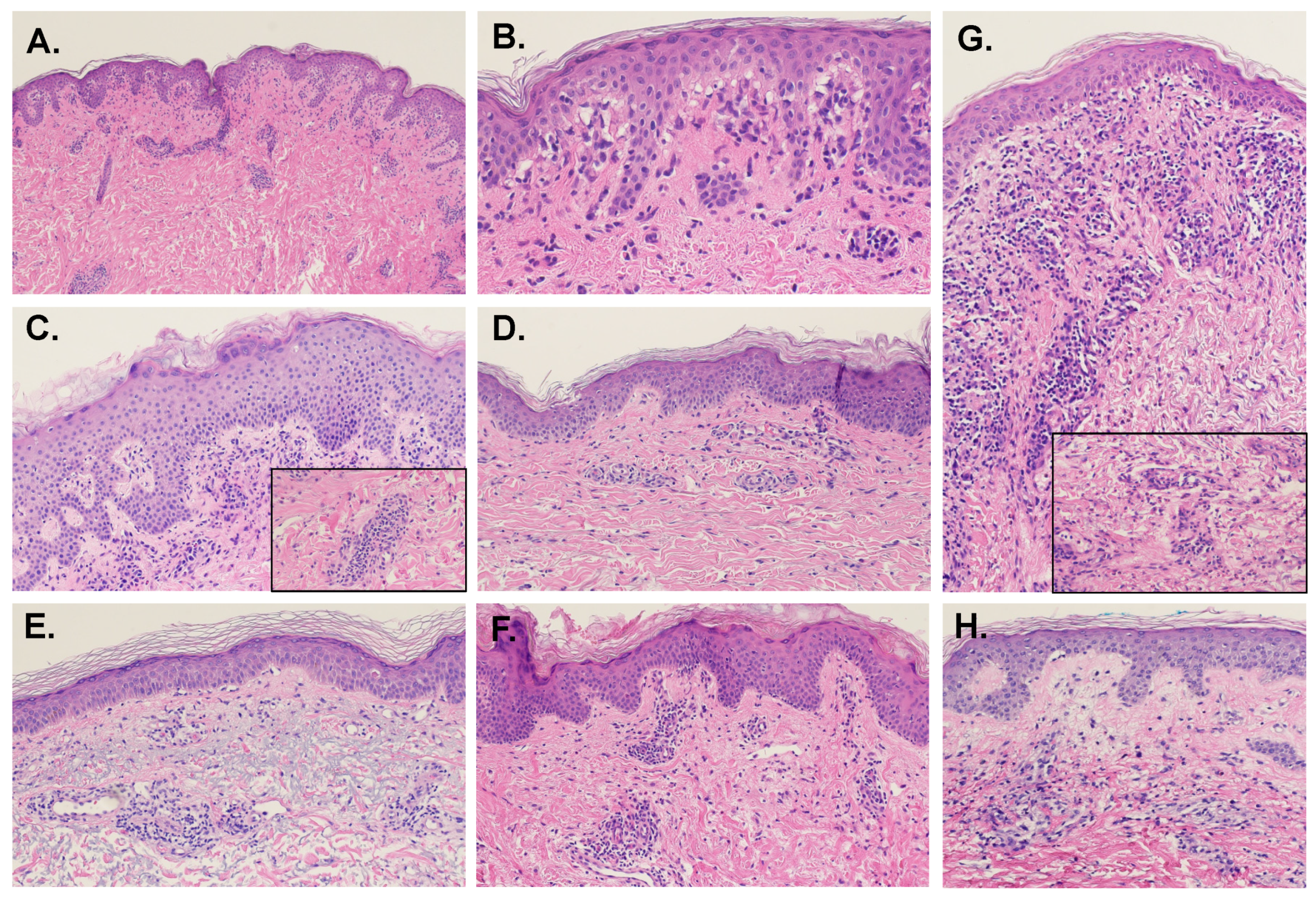
5.1. Epidermal Features
5.2. Dermal-Epidermal Junction Features
5.3. Dermal Features
5.4. Connective Tissue and Vasculature
5.5. Presence of Eosinophils
6. Variants of Maculopapular Drug Eruption
6.1. MDE with Urticarial Aspect
6.2. MDE with Granulomatous Change
6.3. MDE Due to Targeted and Other Oncologic Therapies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ellis, A.; Billings, S.D.; Khanna, U.; Warren, C.B.; Piliang, M.; Vij, A.; Ko, J.S.; Bergfeld, W.F.; Fernandez, A.P. Diagnoses of hospitalized patients with skin abnormalities prompting biopsy by consulting dermatologists: A 3-year review from a tertiary care center. J. Cutan. Pathol. 2020, 47, 346–356. [Google Scholar] [CrossRef]
- Liao, P.; Shih, C.; Mao, C.; Deng, S.; Hsieh, M.; Hsu, K. The cutaneous adverse drug reactions: Risk factors, prognosis and economic impacts. Int. J. Clin. Pract. 2013, 67, 576–584. [Google Scholar] [CrossRef]
- Roujeau, J.C.; Stern, R.S. Severe adverse cutaneous reactions to drugs. N. Engl. J. Med. 1994, 331, 1272–1285. [Google Scholar] [CrossRef]
- Apaydin, R.; Bilen, N.; Dökmeci, S.; Bayramgürler, D.; Yildirim, G. Drug eruptions: A study including all inpatients and outpatients at a dermatology clinic of a university hospital. J. Eur. Acad. Derm. Venereol. 2000, 14, 518–520. [Google Scholar] [CrossRef]
- Bigby, M.; Jick, S.; Jick, H.; Arndt, K. Drug-induced cutaneous reactions. A report from the Boston Collaborative Drug Surveillance Program on 15,438 consecutive inpatients, 1975 to 1982. JAMA 1986, 256, 3358–3363. [Google Scholar] [CrossRef]
- Weyers, W.; Metze, D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Derm. Pract. Concept. 2011, 1, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Ukoha, U.T.; Pandya, A.G.; Dominguez, A.R. Morbilliform Drug Eruptions. In Cutaneous Drug Eruptions: Diagnosis, Histopathology and Therapy; Hall, J.C., Hall, B.J., Eds.; Springer: London, UK, 2015; pp. 45–53. [Google Scholar]
- Naim, M.; Weyers, W.; Metze, D. Histopathologic features of exanthematous drug eruptions of the macular and papular type. Am. J. Derm. 2011, 33, 695–704. [Google Scholar] [CrossRef]
- Stern, R.S. Exanthematous Drug Eruptions. N. Engl. J. Med. 2012, 366, 2492–2501. [Google Scholar] [CrossRef]
- Pichler, W.J.; Naisbitt, D.J.; Park, B.K. Immune pathomechanism of drug hypersensitivity reactions. J. Allergy Clin. Immunol. 2011, 127, S74–S81. [Google Scholar] [CrossRef]
- Bronnimann, M.; Yawalkar, N. Histopathology of drug-induced exanthems: Is there a role in diagnosis of drug allergy? Curr. Opin. Allergy Clin. Immunol. 2005, 5, 317–321. [Google Scholar] [CrossRef]
- Czarnobilska, E.; Obtułowicz, K.; Wsołek, K. [Type IV of hypersensitivity and its subtypes]. Przegl. Lek. 2007, 64, 506–508. [Google Scholar] [PubMed]
- Formica, D.; Sultana, J.; Cutroneo, P.M.; Lucchesi, S.; Angelica, R.; Crisafulli, S.; Ingrasciotta, Y.; Salvo, F.; Spina, E.; Trifirò, G. The economic burden of preventable adverse drug reactions: A systematic review of observational studies. Expert Opin. Drug Saf. 2018, 17, 681–695. [Google Scholar] [CrossRef]
- Neuman, M.G.; McKinney, K.K.; Nanau, R.M.; Kong, V.; Malkiewicz, I.; Mazulli, T.; Moussa, G.; Cohen, L.B. Drug-induced severe adverse reaction enhanced by human herpes virus-6 reactivation. Transl. Res. 2013, 161, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, J.C.; Nanau, R.M.; Neuman, M.G. The Link between Hypersensitivity Syndrome Reaction Development and Human Herpes Virus-6 Reactivation. Int. J. Hepatol. 2012, 2012, 723062. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-I.; Chung, W.-H.; Jee, S.-H.; Chen, W.-C.; Chang, Y.-T.; Lee, W.-R.; Hu, S.-L.; Wu, M.-T.; Chen, G.-S.; Wong, T.-W.; et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharm. Genom. 2006, 16, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Rehm, C.D.; Haas, J.S.; Chan, A.T.; Giovannucci, E.L. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA 2015, 314, 1818–1831. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khandpur, S.; Arava, S.; Rath, R.; Ramam, M.; Singh, M.; Sharma, V.K.; Kabra, S.K. Assessment of histopathological features of maculopapular viral exanthem and drug-induced exanthem. J. Cutan. Pathol. 2017, 44, 1038–1048. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.; Janecek, E.; Domecq, C.; Greenblatt, D. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Ardern-Jones, M.R.; Mockenhaupt, M. Making a diagnosis in severe cutaneous drug hypersensitivity reactions. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 283–293. [Google Scholar] [CrossRef]
- Ackerman, A.B.; Chongchitnant, N.; Sanchez, J.; Guo, Y.; Bennin, B.; Reichel, M.; Randall, M. Histologic Diagnosis of Inflammatory Skin Disease: An Algorithm Method Based on Pattern Analysis, 2nd ed.; Williams and Walkins: Baltimore, MD, USA, 1997. [Google Scholar]
- Bolognia, J.; Schaffer, J.; Cerroni, L. Dermatology, 4th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Gerson, D.; Sriganeshan, V.; Alexis, J.B. Cutaneous drug eruptions: A 5-year experience. J. Am. Acad. Dermatol. 2008, 59, 995–999. [Google Scholar] [CrossRef]
- Rapini, R. Practical Dermatopathology, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2021. [Google Scholar]
- Crowson, A.N.; Brown, T.J.; Magro, C.M. Progress in the Understanding of the Pathology and Pathogenesis of Cutaneous Drug Eruptions. Am. J. Clin. Dermatol. 2003, 4, 407–428. [Google Scholar] [CrossRef] [PubMed]
- Justiniano, H.; Berlingeri-Ramos, A.C.; Sánchez, J.L. Pattern analysis of drug-induced skin diseases. Am. J. Derm. 2008, 30, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Kwon, J.-E.; Han, J.-H.; Kim, Y.-C. Histopathologic Study of Morbilliform Drug Eruption: Differences between Chemotherapeutic Agents and Antibiotics. Korean J. Dermatol. 2018, 56, 368–375. [Google Scholar]
- Weinborn, M.; Barbaud, A.; Truchetet, F.; Beurey, P.; Germain, L.; Cribier, B. Histopathological study of six types of adverse cutaneous drug reactions using granulysin expression. Int. J. Dermatol. 2016, 55, 1225–1233. [Google Scholar] [CrossRef]
- Fellner, M.J.; Prutkin, L. Morbilliform Eruptions Caused by Penicillin**From the Departments of Dermatology and Cell. Biology, New York University School of Medicine, New York, New York. J. Investig. Dermatol. 1970, 55, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Ruiz, S.; Penas, P.; Fernández-Herrera, J.; Sánchez-Pérez, J.; Fraga, J.; García-Díez, A. Maculopapular eruption with enlarged macrophages in eight patients receiving G-CSF or GM-CSF. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 310–313. [Google Scholar] [CrossRef]
- Valks, R.; Vargas, E.; Muñoz, E.; Fernández-Herrera, J.; Garcia-Diéz, A.; Fraga, J. Dermal infiltrate of enlarged macrophages in patients receiving chemotherapy. J. Cutan. Pathol. 1998, 25, 259–264. [Google Scholar] [CrossRef]
- Bellini, V.; Pelliccia, S.; Lisi, P. Drug- and Virus- or Bacteria-induced Exanthems: The Role of Immunohistochemical Staining for Cytokines in Differential Diagnosis. Dermatitis 2013, 24, 85–90. [Google Scholar] [CrossRef]
- Ortonne, N.; Valeyrie-Allanore, L.; Bastuji-Garin, S.; Wechsler, J.; de Feraudy, S.; Duong, T.-A.; Delfau-Larue, M.-H.; Chosidow, O.; Wolkenstein, P.; Roujeau, J.-C. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: A morphological and phenotypical study. Br. J. Derm. 2015, 173, 50–58. [Google Scholar] [CrossRef]
- Wang, E.C.; Lee, J.S.; Tan, A.W.; Tang, M.B. Fas-ligand staining in non-drug-and drug-induced maculopapular rashes. J. Cutan. Pathol. 2011, 38, 196–201. [Google Scholar] [CrossRef]
- Nghiem, P. The “drug vs graft-vs-host disease” conundrum gets tougher, but there is an answer: The challenge to dermatologists. Arch. Derm. 2001, 137, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Billings, S.; Cotton, J. Inflammatory Dermatopathology: A Pathologist’s Survival Guide; Springer Science+Business Media, LLC: Berlin, Germany, 2011. [Google Scholar]
- LeBoit, P.E. Interface dermatitis. How specific are its histopathologic features? Arch. Derm. 1993, 129, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.L.; Torres-Cabala, C.A.; Kim, K.B.; Tetzlaff, M.T.; Duvic, M.; Tsai, K.Y.; Hong, D.S.; Prieto, V.G. Dermatologic toxicities to targeted cancer therapy: Shared clinical and histologic adverse skin reactions. Int. J. Dermatol. 2014, 53, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Marra, D.E.; McKee, P.H.; Nghiem, P. Tissue eosinophils and the perils of using skin biopsy specimens to distinguish between drug hypersensitivity and cutaneous graft-versus-host disease. J. Am. Acad Derm. 2004, 51, 543–546. [Google Scholar] [CrossRef]
- Weaver, J.; Bergfeld, W.F. Quantitative analysis of eosinophils in acute graft-versus-host disease compared with drug hypersensitivity reactions. Am. J. Derm. 2010, 32, 31–34. [Google Scholar] [CrossRef]
- Stevens, A.; Dalziel, K. The histopathology of drug rashes. Curr. Diagn. Pathol. 1998, 5, 138–149. [Google Scholar] [CrossRef]
- Horn, T.D.; Burke, P.J.; Karp, J.E.; Hood, A.F. Intravenous Administration of Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor Causes a Cutaneous Eruption. Arch. Dermatol. 1991, 127, 49–52. [Google Scholar] [CrossRef]
- Macdonald, J.B.; Macdonald, B.; Golitz, L.E.; LoRusso, P.; Sekulic, A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J. Am. Acad. Derm. 2015, 72, 203–218. [Google Scholar] [CrossRef]
- Ransohoff, J.D.; Kwong, B.Y. Cutaneous adverse events of targeted therapies for hematolymphoid malignancies. Clin. Lymphoma Myeloma Leuk. 2017, 17, 834–851. [Google Scholar] [CrossRef]
- Valeyrie, L.; Bastuji-Garin, S.; Revuz, J.; Bachot, N.; Wechsler, J.; Berthaud, P.; Tulliez, M.; Giraudier, S. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: A prospective study of 54 patients. J. Am. Acad. Dermatol. 2003, 48, 201–206. [Google Scholar] [CrossRef]
- Sinha, R.; Edmonds, K.; Newton-Bishop, J.A.; Gore, M.E.; Larkin, J.; Fearfield, L. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: Practical advice on diagnosis, prevention and management of the main treatment-related skin toxicities. Br. J. Derm. 2012, 167, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; De Souza, E.; Cintra, M.; Pagnano, K.; Chiari, A.; Lorand-Metze, I. Cutaneous adverse reaction to 2-chlorodeoxyadenosine with histological flame figures in patients with chronic lymphocytic leukaemia. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Soefje, S.A.; Karnad, A.; Brenner, A.J. Common toxicities of mammalian target of rapamycin inhibitors. Target. Oncol. 2011, 6, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Alexandre, J.; Faivre, S.; Vera, K.; Materman, E.; Boni, J.; Leister, C.; Korth-Bradley, J.; Hanauske, A.; Armand, J.-P. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J. Clin. Oncol. 2004, 22, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Torres, L.; Llamas-Velasco, M.; Machan, S.; Haro, R.; De Asis, S.; Carmo, M.; Loredo, A.; Del Puerto, C.; Fried, I.; Kempf, W. Taxanes-induced cutaneous eruption: Another histopathologic mimicker of malignancy. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 638–644. [Google Scholar] [CrossRef]
| Feature | Weinborn et al. [29] n = 33 n (%) | Naim et al. [8] n = 60 n (%) | Cho et al. [28] n = 40 n (%) | Gerson et al. [23] n = 104 n (%) | Bellini et al. [32] n = 36 n (%) | Ortonne et al. [33] n= 20 n (%) | Wang et al. [34] n = 10 n (%) | Signh et al. [18] n = 24 n (%) | Overall n = 327 % |
|---|---|---|---|---|---|---|---|---|---|
| Epidermis | |||||||||
| Hyperplasia | - | 43 (72) | 18 (45) | - | 9 (25) | - | - | 5 (21) | 47 |
| Basket-weave orthokeratosis | - | - | 19 (48) | - | 23 (64) | - | - | - | 55 |
| Compact orthokeratosis | - | 8 (13) | - | - | - | - | - | 2 (8.3) | 12 |
| Scale crust | - | 5 (8) | - | - | - | - | - | - | 8 |
| Parakeratosis (any) | 6 (18) | 10 (17) | 5 (13) | - | 3 (8.3) | 8 (40) | - | 2 (8.3) | 16 |
| Focal | - | 8 (13) | - | - | 3 (8.3) | - | - | - | 11 |
| Compact | - | 2 (3) | - | - | - | - | - | - | 3 |
| Spongiosis present (any) | 23 (70) | 58 (97) | 21 (53) | - | 22 (61) | - | - | 12 (50) | 70 |
| Lower only | - | 47 (78) | - | - | - | - | - | - | 78 |
| All Layers | - | 11 (18) | - | - | - | - | - | - | 18 |
| Focal | - | 27 (45) | - | - | - | - | - | 12 (50) | 46 |
| Continuous | - | 31 (52) | - | - | - | - | - | 52 | |
| Inflammatory infiltrate (any) | 14 (42) | 60 (100) | 27 (68) | - | 17 (47) | 7 (35) | 9 (90) | 14 (58) | 66 |
| Lymphocytic | - | 49 (82) | 25 (63) | - | 17 (47) | 7 (35) | - | 14 (58) | 62 |
| Neutrophilic | - | 19 (32) | 2 (5) | - | 7 (19) | - | - | 1 (4.2) | 18 |
| Eosinophilic | 0 | 2 (3) | 3 (7.5) | - | - | - | - | 2 (8.3) | 4 |
| Erythrocytes | - | 5 (8) | - | - | - | - | - | 2 (8.3) | 16 |
| Necrotic keratinocytes | 8 (24) | 13 (22) | 35 (88) | - | - | - | - | 5 (21) | 39 |
| Atrophy | 8 (24) | - | - | - | - | - | - | - | 24 |
| Mitoses | - | 20 (33) | - | - | - | - | - | 2 (8.3) | 26 |
| DEJ | |||||||||
| Basal vacuolization/ interface change (any) | 9 (27) | 58 (97) | 38 (95) | 54 (52) | 11 (31) | 7 (35) | 7 (70) | 7 (29) | 58 |
| Focal | - | 26 (43) | 29 (73) | - | - | 7 (35) | - | 6 (25) | 47 |
| Continuous | - | 32 (53) | 9 (23) | - | - | - | - | 1 (4.2) | 34 |
| Dermis | |||||||||
| Edema (superficial) | 20 (61) | 51 (85) | 26 (65) | - | 26 (73) | 7 (35) | - | - | 69 |
| Edema (deep) | - | 28 (47) | 1 (2.5) | - | 11 (29) | - | - | - | 43 |
| Infiltrate (any) | 33 (100) | 60 (100) | 40 (100) | 102 (98) | 33 (92) | 20 (100) | - | 15 (62) | 96 |
| Superficial (any) | 33 (100) | 60 (100) | 40 (100) | 97 (95) | - | 20 (100) | - | - | 97 |
| Deep (any) | 9 (27) | 29 (48) | 2 (5) | 5 (5) | - | 1 (5) | - | - | 18 |
| Perivascular (any) | - | 60 (100) | - | 102 (98) | 33 (92) | 8 (40) | - | 12 (50) | 88 |
| Superficial Perivascular (any) | - | 43 (72) | - | - | - | - | - | - | 72 |
| Deep Perivascular (any) | - | 17 (28) | - | - | - | - | - | - | 28 |
| Interstitial (all) | - | 56 (93) | - | 82 (80) | - | - | - | 13 (54) | 80 |
| Superficial | - | 56 (93) | - | - | - | - | - | - | 93 |
| Deep | - | 29 (48) | - | - | - | - | - | - | 48 |
| Lymphocytic (all) | - | 60 (100) | 40 (100) | 95 (91) | - | 20 (100) | - | 14 (58) | 92 |
| Perivascular | - | 60 (100) | - | - | - | - | - | 12 (50) | 86 |
| Interstitial | - | 53 (88) | - | - | - | - | - | 2 (8.3) | 65 |
| Superficial | - | 60 (100) | - | - | - | - | - | 14 (58) | 88 |
| Deep | - | 29 (48) | - | - | - | - | - | - | 48 |
| Eosinophilic (all) | 20 (61) | 46 (77) | 27 (68) | 52 (50) | 13 (36) | 9 (45) | 6 (60) | 15 (62) | 57 |
| Perivascular | - | 36 (60) | - | - | - | - | - | 7 (29) | 51 |
| Interstitial | - | 33 (55) | - | - | - | - | - | 13 (54) | 55 |
| Superficial | 20 (61) | 36 (60) | - | - | - | - | - | 2 (8.3) | 60 |
| Deep | 4 (12) | 46 (77) | - | - | - | - | - | - | 54 |
| Neutrophilic (all) | - | 38 (63) | 11 (28) | 37 (36) | - | 6 (30) | - | 2 (8.3) | 41 |
| Perivascular | - | 30 (50) | - | - | - | - | - | 1 (4.2) | 50 |
| Interstitial | - | 46 (77) | - | - | - | - | - | 1 (4.2) | 77 |
| Superficial | - | 46 (77) | - | - | - | - | - | 1 (4.2) | 77 |
| Deep | - | 38 (63) | - | - | - | - | - | - | 63 |
| Macrophages | - | 39 (65) | - | - | - | - | - | - | 65 |
| Erythrocytes | - | 17 (28) | - | - | - | - | - | - | 28 |
| Common (>55%) | Less Common (26–55%) | Atypical Findings (<25%) | |
|---|---|---|---|
| Epidermis | Focal or continuous spongiosis of lower epidermis without vesiculation | Mild, regular hyperplasia | Discrete mounds of parakeratosis |
| Mild lymphocytic infiltrate | Necrotic keratinocytes | Compact orthokeratosis | |
| Regular basket-weave orthokeratosis | Mitoses | Increased eosinophils, neutrophils, melanophages, or erythrocytes | |
| Pronounced epidermal damage | |||
| Scale crust | |||
| Satellite cell necrosis | |||
| Langerhans cell microabscesses | |||
| DEJ | Basal vacuolization/interface change (focal or continuous) | Necrotic keratinocytes | |
| Dermis | Superficial perivascular and interstitial lymphocytic and eosinophilic infiltrate +/− neutrophils | Erythrocyte extravasation | Colloid bodies |
| Macrophages without granuloma formation | Deep, interstitial and perivascular infiltrate | Atypical lymphocytes | |
| Increased mast cells | |||
| Connective tissue and vessels | Mild edema of papillary dermis | Vasculitis/leukocytoclasia | |
| Dilation of superficial dermal lymph and blood vessels | Fibrosis | ||
| Interstitial mucin deposits |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernst, M.; Giubellino, A. Histopathologic Features of Maculopapular Drug Eruption. Dermatopathology 2022, 9, 111-121. https://doi.org/10.3390/dermatopathology9020014
Ernst M, Giubellino A. Histopathologic Features of Maculopapular Drug Eruption. Dermatopathology. 2022; 9(2):111-121. https://doi.org/10.3390/dermatopathology9020014
Chicago/Turabian StyleErnst, Madison, and Alessio Giubellino. 2022. "Histopathologic Features of Maculopapular Drug Eruption" Dermatopathology 9, no. 2: 111-121. https://doi.org/10.3390/dermatopathology9020014
APA StyleErnst, M., & Giubellino, A. (2022). Histopathologic Features of Maculopapular Drug Eruption. Dermatopathology, 9(2), 111-121. https://doi.org/10.3390/dermatopathology9020014







