Artificial Intelligence in Dermatopathology: New Insights and Perspectives
Abstract
:1. Introduction
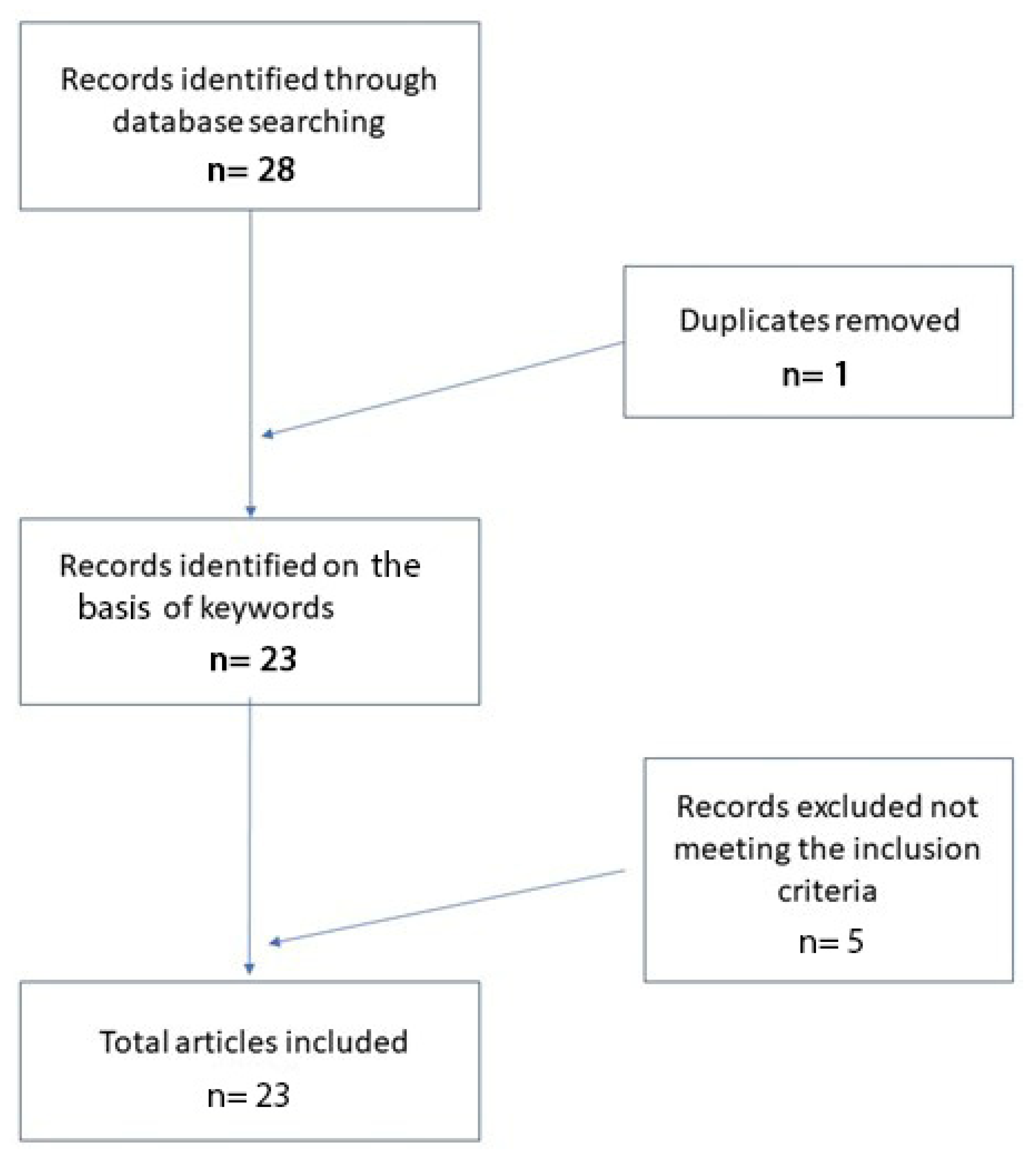
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tizhoosh, H.R.; Pantanowitz, L. Artificial Intelligence and Digital Pathology: Challenges and Opportunities. J. Pathol. Inform. 2018, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [Green Version]
- Wells, A.; Patel, S.; Lee, J.B.; Motaparthi, K. Artificial intelligence in dermatopathology: Diagnosis, education, and research. J. Cutan. Pathol. 2021, 48, 1061–1068. [Google Scholar] [CrossRef]
- Bauer, T.W.; Schoenfield, L.; Slaw, R.J.; Yerian, L.; Sun, Z.; Henricks, W.H. Validation of whole slide imaging for primary diagnosis in surgical pathology. Arch. Pathol. Lab. Med. 2013, 137, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Venerito, V.; Angelini, O.; Cazzato, G.; Lopalco, G.; Maiorano, E.; Cimmino, A.; Iannone, F. A convolutional neural network with transfer learning for automatic discrimination between low and high-grade synovitis: A pilot study. Intern Emerg. Med. 2021, 16, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Liguori, G.; Lettini, T.; Serio, G.; Ingravallo, G.; Marzullo, A. Atypical Fibroxanthoma-Like Amelanotic Melanoma: A Diagnostic Challenge. Dermatopathology 2021, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Onega, T.; Reisch, L.M.; Frederick, P.D.; Geller, B.M.; Nelson, H.D.; Lott, J.P.; Radick, A.C.; Elder, D.E.; Barnhill, R.L.; Piepkorn, M.W.; et al. Use of digital whole slide imaging in dermatopathology. J. Digit. Imaging 2016, 29, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Polesie, S.; McKee, P.H.; Gardner, J.M.; Gillstedt, M.; Siarov, J.; Neittaanmäki, N.; Paoli, J. Attitudes Toward Artificial Intelligence Within Dermatopathology: An International Online Survey. Front. Med. 2020, 20, 591952. [Google Scholar] [CrossRef]
- Liu, X.; Faes, L.; Kale, A.U.; Wagner, S.K.; Fu, D.J.; Bruynseels, A.; Mahendiran, T.; Moraes, G.; Shamdas, M.; Kern, C.; et al. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: A systematic review and meta-analysis. Lancet Digit. Health 2019, 1, e271–e297. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, N.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Bengio, Y.; Goodfellow, I.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Gomolin, A.; Netchiporouk, E.; Gniadecki, R.; Litvinov, I.V. Artificial Intelligence Applications in Dermatology: Where Do We Stand? Front. Med. (Lausanne) 2020, 31, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hekler, A.; Utikal, J.S.; Enk, A.H.; Berking, C.; Klode, J.; Schadendorf, D.; Jansen, P.; Franklin, C.; Holland-Letz, T.; Krahl, D.; et al. Pathologist-level classification of histopathological melanoma images with deep neural networks. Eur. J. Cancer 2019, 115, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.Q.; Xiong, J.H.; Li, H.Y.; Yang, X.H.; Yu, W.T.; Gao, M.; Zhao, X.; Ma, Y.P.; Zhang, W.; Guan, Y.F.; et al. Recognizing basal cell carcinoma on smartphone-captured digital histopathology images with a deep neural network. Br. J. Dermatol. 2020, 182, 754–762. [Google Scholar] [CrossRef]
- Cruz-Roa, A.A.; Arevalo Ovalle, J.E.; Madabhushi, A.; González Osorio, F.A. A deep learning architecture for image representation, visual interpretability and automated basal-cell carcinoma cancer detection. Med. Image Comput. Comput. Assist Interv. 2013, 16, 403–410. [Google Scholar]
- Potter, B.; Ronan, S.G. Computerized dermatopathologic diagnosis. J. Am. Acad. Dermatol. 1987, 17, 119–131. [Google Scholar] [CrossRef]
- Crowley, R.S.; Medvedeva, O. A general architecture for intelligent tutoring of diagnostic classification problem solving. AMIA Annu. Symp. Proc. 2003, 2003, 185–189. [Google Scholar]
- Feit, J.; Kempf, W.; Jedlicková, H.; Burg, G. Hypertext atlas of dermatopathology with expert system for epithelial tumors of the skin. J. Cutan. Pathol. 2005, 32, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Payne, V.L.; Medvedeva, O.; Legowski, E.; Castine, M.; Tseytlin, E.; Jukic, D.; Crowley, R.S. Effect of a limited-enforcement intelligent tutoring system in dermatopathology on student errors, goals and solution paths. Artif. Intell. Med. 2009, 47, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.G.; Jackson, B.H.; Feeser, T.A.; Kent, M.N.; Moad, J.C.; Krishnamurthy, S.; Lunsford, D.D.; Soans, R.E. Diagnostic Performance of Deep Learning Algorithms Applied to Three Common Diagnoses in Dermatopathology. J. Pathol. Inform. 2018, 27, 32. [Google Scholar]
- Peizhen, I.; Xie, K.; Zuo, Y.; Zhang, F.; Li, M.; Yin, K. Interpretable Classification from Skin Cancer Histology Slides Using Deep Learning: A Retrospective Multicenter Study. arXiv 2019, arXiv:1904.06156. [Google Scholar]
- Goyal, M.; Knackstedt, T.; Yan, S.; Hassanpour, S. Artificial intelligence-based image classification methods for diagnosis of skin cancer: Challenges and opportunities. Comput. Biol. Med. 2020, 127, 104065. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21, 5394. [Google Scholar] [CrossRef] [PubMed]

| Authors | Years | Type of AI | Results | Strengths | Limits |
|---|---|---|---|---|---|
| Potter et al. [16] | 1987 | Interactive computer program | Concordance, 91.8% Disagreement, 4.8% | Concordance and possibility of integration with patient clinical data | Disagreement and little memory space |
| Crowlet R. et al. [17] | 2003 | Traditional intelligent tutoring system | Possibility of learning rather easily | Positive feedback | Clear prototypical schemes are indispensable |
| Joset Feit et al. [18] | 2005 | Hypertext atlas of dermatopathology | A collection of about 3200 dermatopathological images | Continuous updating | / |
| Payne et al. [19] | 2009 | Intelligent tutoring system | Tutoring made it possible to implement the training of learners | Ability to learn from mistakes | Greater difficulties in tutoring related to superficial perivascular dermatitis |
| Olsen et al. [20] | 2018 | Deep learning algorithms | The artificial intelligence system accurately classified 123/124 (99.45%) BCCs (nodular), 113/114 (99.4%) dermal nevi and 123/123 (100%) seborrheic keratoses | Concordance | Difficulty in presenting artifacts, poor coloring |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, G.; Colagrande, A.; Cimmino, A.; Arezzo, F.; Loizzi, V.; Caporusso, C.; Marangio, M.; Foti, C.; Romita, P.; Lospalluti, L.; et al. Artificial Intelligence in Dermatopathology: New Insights and Perspectives. Dermatopathology 2021, 8, 418-425. https://doi.org/10.3390/dermatopathology8030044
Cazzato G, Colagrande A, Cimmino A, Arezzo F, Loizzi V, Caporusso C, Marangio M, Foti C, Romita P, Lospalluti L, et al. Artificial Intelligence in Dermatopathology: New Insights and Perspectives. Dermatopathology. 2021; 8(3):418-425. https://doi.org/10.3390/dermatopathology8030044
Chicago/Turabian StyleCazzato, Gerardo, Anna Colagrande, Antonietta Cimmino, Francesca Arezzo, Vera Loizzi, Concetta Caporusso, Marco Marangio, Caterina Foti, Paolo Romita, Lucia Lospalluti, and et al. 2021. "Artificial Intelligence in Dermatopathology: New Insights and Perspectives" Dermatopathology 8, no. 3: 418-425. https://doi.org/10.3390/dermatopathology8030044
APA StyleCazzato, G., Colagrande, A., Cimmino, A., Arezzo, F., Loizzi, V., Caporusso, C., Marangio, M., Foti, C., Romita, P., Lospalluti, L., Mazzotta, F., Cicco, S., Cormio, G., Lettini, T., Resta, L., Vacca, A., & Ingravallo, G. (2021). Artificial Intelligence in Dermatopathology: New Insights and Perspectives. Dermatopathology, 8(3), 418-425. https://doi.org/10.3390/dermatopathology8030044












