TP53 Abnormalities and MMR Preservation in 5 Cases of Proliferating Trichilemmal Tumours
Abstract
1. Introduction
2. Materials and Methods
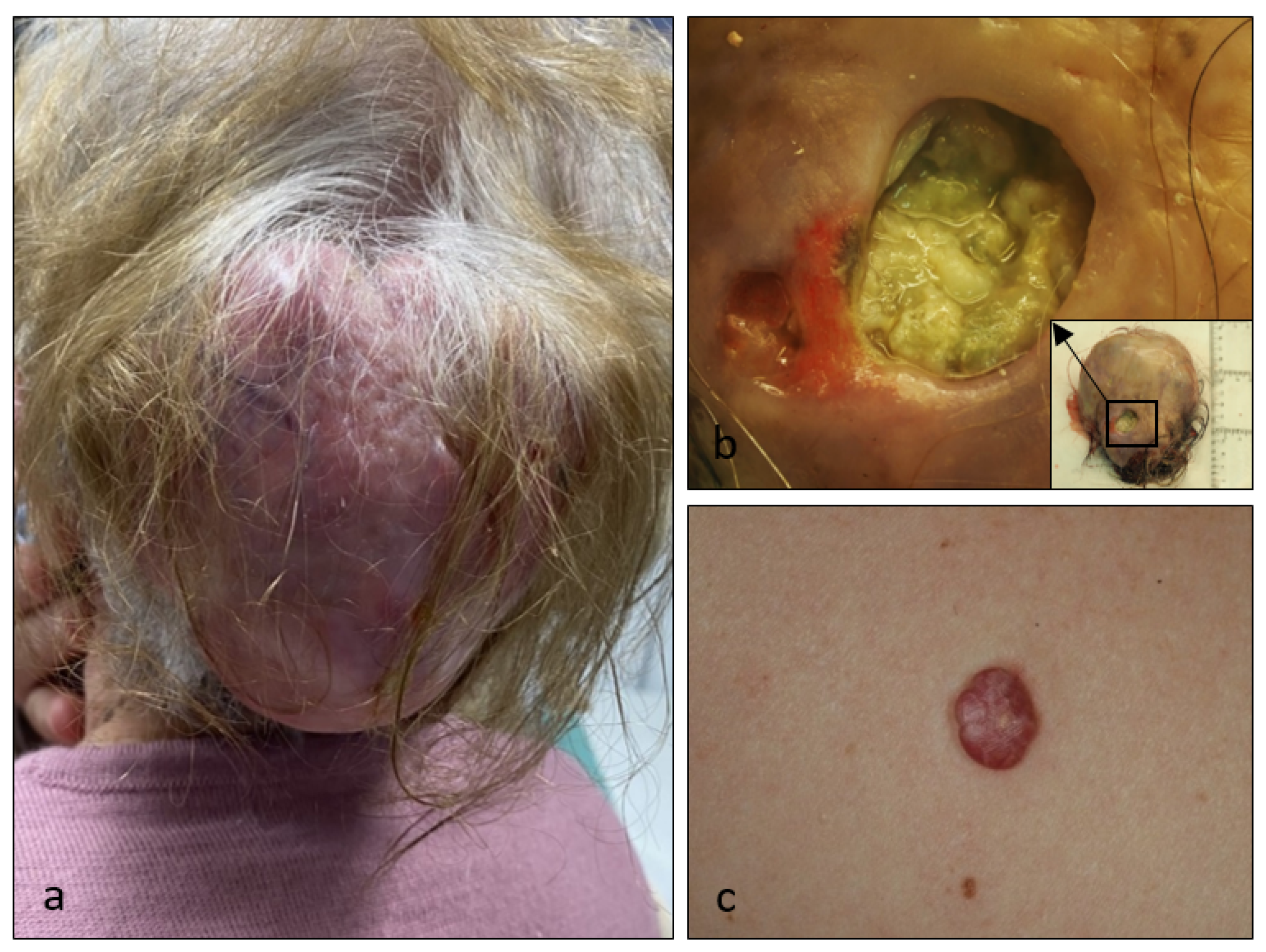
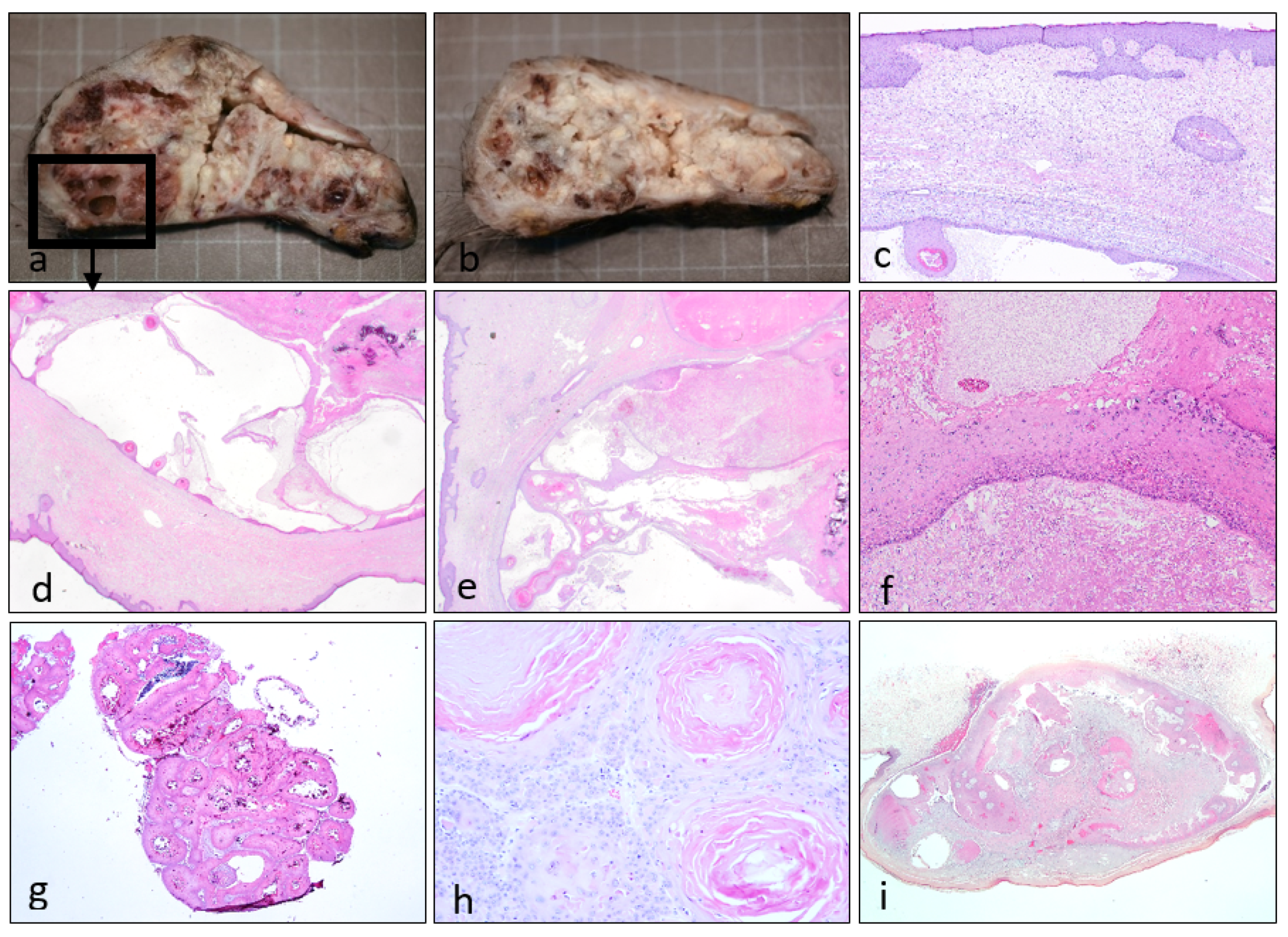
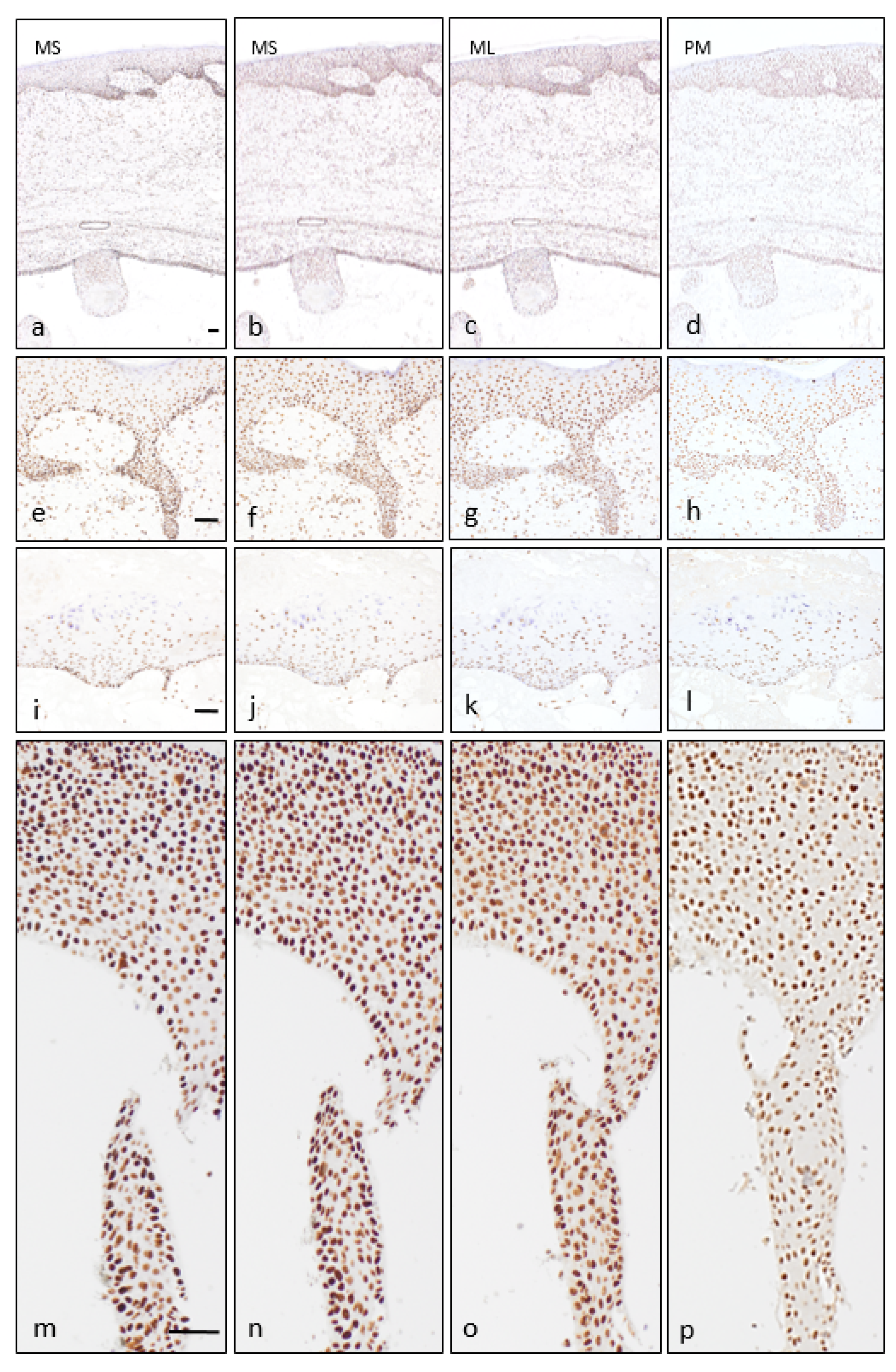
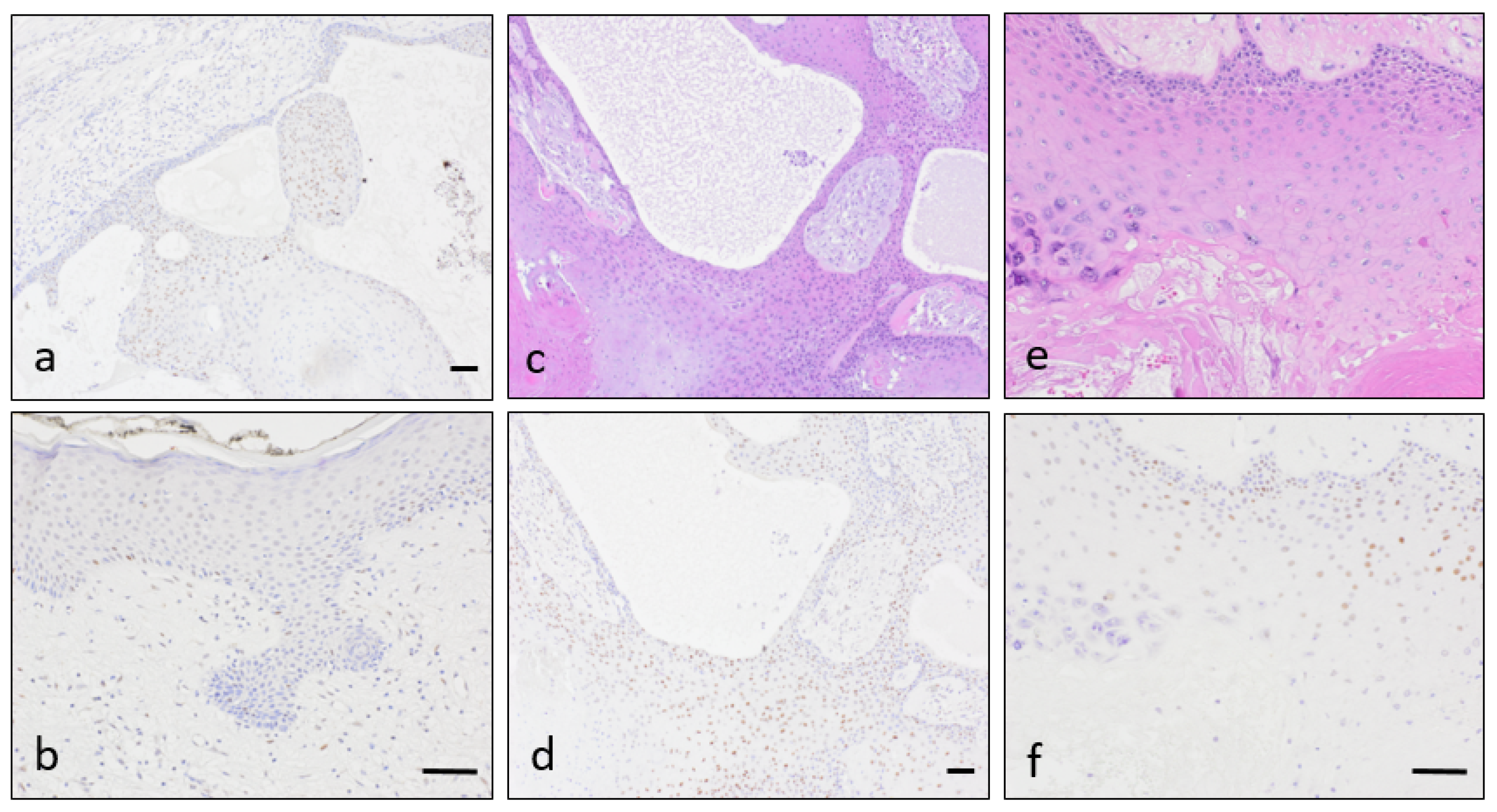
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pinkus, H. “Sebaceous cysts” are trichilemmal cysts. Arch. Dermatol. 1969, 99, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Kamyab, K.; Kianfar, N.; Dasdar, S.; Salehpour, Z.; Nasimi, M. Cutaneous cysts: A clinicopathologic analysis of 2438 cases. Int. J. Dermatol. 2020, 59, 457–462. [Google Scholar] [CrossRef]
- Leppard, B.J.; Sanderson, K.; Wells, R. Hereditary trichilemmal cysts. Hereditary pilar cysts. Clin. Exp. Dermatol. 1977, 2, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Satyaprakash, A.K.; Sheehan, D.J.; Sangueza, O.P. Proliferating trichilemmal tumors: A review of the literature. Dermatol. Surg. 2007, 33, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.W. Proliferating epidermoid cysts. Arch. Dermatol. 1966, 94, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Noto, G. ‘Benign’ proliferating trichilemmal tumour: Does it really exist? Histopathology 1999, 35, 386–387. [Google Scholar] [CrossRef]
- Ye, J.; Nappi, O.; Swanson, P.E.; Patterson, J.W.; Wick, M.R. Proliferating pilar tumors: A clinicopathologic study of 76 cases with a proposal for definition of benign and malignant variants. Am. J. Clin. Pathol. 2004, 122, 566–574. [Google Scholar] [CrossRef]
- Baptista, A.P.; e Silva, L.G.; Born, M.C. Proliferating trichilemmal cyst. J. Cutan. Pathol. 1983, 10, 178–187. [Google Scholar] [CrossRef]
- Bhavya, P.M.; Letha, V.; Anilkumar, V. Malignant Proliferating Trichilemmal Tumor: A Rare Adnexal Neoplasm. Indian J. Dermatopathol. Diagn. Dermatol. 2020, 7, 40–41. [Google Scholar] [CrossRef]
- Park, B.S.; Yang, S.G.; Cho, K.H. Malignant Proliferating Trichilemmal Tumor Showing Distant Metastases. Am. J. Dermatopathol. 1997, 19, 536–539. [Google Scholar] [CrossRef]
- Sau, P.; Graham, J.H.; Helwig, E.B. Proliferating epithelial cysts. Clinicopathological analysis of 96 cases. J. Cutan. Pathol. 1995, 22, 394–406. [Google Scholar] [CrossRef]
- Takata, M.; Rehman, I.; Rees, J.L. A trichilemmal carcinoma arising from a proliferating trichilemmal cyst: The loss of the wild-type p53 is a critical event in malignant transformation. Hum. Pathol. 1998, 29, 193–195. [Google Scholar] [CrossRef]
- Singh, P.; Usman, A.; Motta, L.; Khan, I. Malignant proliferating trichilemmal tumour. BMJ Case Rep. 2018, 2018, 224460. [Google Scholar] [CrossRef] [PubMed]
- Chaichamnan, K.; Satayasoontorn, K.; Puttanupaab, S.; Attainsee, A. Malignant proliferating trichilemmal tumors with CD34 expression. J. Med. Assoc. Thail. 2010, 93, S28–S34. [Google Scholar]
- Vargas-Mora, P.; Orlandi, D.; Morales, C.; Araya, I. Proliferating Trichilemmal Cysts: A Clinicopathological Study of 14 Cases. Int. J. Trichol. 2019, 11, 258–259. [Google Scholar] [CrossRef]
- Haas, N.; Audring, H.; Sterry, W. Carcinoma arising in a proliferating trichilemmal cyst expresses fetal and trichilemmal hair phenotype. Am. J. Dermatopathol. 2002, 24, 340–344. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Matsuo, S.; Iizuka, H. A DNA-flow cytometric analysis of trichilemmal carcinoma, proliferating trichilemmal cyst and trichilemmal cyst. Acta Dermato-Venereol. 1994, 74, 358–360. [Google Scholar]
- Takata, M.; Quinn, A.G.; Hashimoto, K.; Rees, J.L. Low Frequency of loss and heterozygosity at the nevoid basal cell carcinoma locus and other selected loci in appendageal tumors. J. Investig. Dermatol. 1996, 106, 1141–1144. [Google Scholar] [CrossRef]
- Fernández-Figueras, M.T.; Casalots, A.; Puig, L.; Llatjós, R.; Ferrándiz, C.; Ariza, A. Proliferating trichilemmal tumor: p53 immunoreactivity in association with p27Kip1 over-expression indicates a low-grade carcinoma profile. Histopathology 2001, 38, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Hörer, S.; Marrakchi, S.; Radner, F.P.; Zolles, G.; Heinz, L.; Eichmann, T.O.; Has, C.; Salavei, P.; Mahfoudh, N.; Turki, H.; et al. A Monoallelic Two-Hit Mechanism in PLCD1 Explains the Genetic Pathogenesis of Hereditary Trichilemmal Cyst Formation. J. Investig. Dermatol. 2019, 139, 2154–2163.e5. [Google Scholar] [CrossRef]
- Kolodney, M.S.; Coman, G.C.; Smolkin, M.B.; Hagen, R.; Katzman, J.A.; Katzman, S.N.; Holliday, A.C.; Kolodney, J.A. Hereditary Trichilemmal Cysts are Caused by Two Hits to the Same Copy of the Phospholipase C Delta 1 Gene (PLCD1). Sci. Rep. 2020, 10, 6035. [Google Scholar] [CrossRef]
- Rutty, G.; Richman, P.; Laing, J. Malignant change in trichilemmal cysts: A study of cell proliferation and DNA content. Histopathology 1992, 21, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Herrero, J.; Monteagudo, C.; Ruiz, A.; Llombart-Bosch, A. Malignant proliferating trichilemmal tumours: An histopathological and immunohistochemical study of three cases with DNA ploidy and morphometric evaluation. Histopathology 1998, 33, 542–546. [Google Scholar] [CrossRef]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar]
- Moreira, L.; Balaguer, F.; Lindor, N.; De La Chapelle, A.; Hampel, H.; Aaltonen, L.A.; Hopper, J.L.; Le Marchand, L.; Gallinger, S.; Newcomb, P.A.; et al. Identification of lynch syndrome among patients with colorectal cancer. JAMA 2012, 308, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Le, S.; Ansari, U.; Mumtaz, A.; Malik, K.; Patel, P.; Doyle, A.; Khachemoune, A. Lynch Syndrome and Muir-Torre Syndrome: An update and review on the genetics, epidemiology, and management of two related disorders. Dermatol. Online J. 2017, 23, 13030. [Google Scholar] [PubMed]
- Kruse, R.; Ruzicka, T. DNA mismatch repair and the significance of a sebaceous skin tumor for visceral cancer prevention. Trends Mol. Med. 2004, 10, 136–141. [Google Scholar] [CrossRef]
- Hall, M.J.; Neumann, C.C.; Lamont, J.T.; Grover, S. Lynch syndrome (hereditary nonpolyposis colorectal cancer): Clinical manifestations and diagnosis. In UpToDate; Post, T.W., Ed.; UpToDate: Waltham, MA, USA, 2020; Available online: https://www.uptodate.com/contents/lynch-syndrome-hereditary-nonpolyposis-colorectal-cancer-clinical-manifestations-and-diagnosis (accessed on 24 May 2021).
- Everett, J.N.; Raymond, V.M.; Dandapani, M.; Marvin, M.; Kohlmann, W.; Chittenden, A.; Koeppe, E.; Gustafson, S.L.; Else, T.; Fullen, D.R.; et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014, 150, 1315–1321. [Google Scholar] [CrossRef]
- Boennelycke, M.; Thomsen, B.M.; Holck, S. Sebaceous neoplasms and the immunoprofile of mismatch-repair proteins as a screening target for syndromic cases. Pathol. Res. Pract. 2015, 211, 78–82. [Google Scholar] [CrossRef]
- Lamba, A.R.; Moore, A.Y.; Moore, T.; Rhees, J.; Arnold, M.A.; Boland, C.R. Defective DNA mismatch repair activity is common in sebaceous neoplasms, and may be an ineffective approach to screen for Lynch syndrome. Fam. Cancer 2015, 14, 259–264. [Google Scholar] [CrossRef]
- Kuwabara, K.; Suzuki, O.; Chika, N.; Kumamoto, K.; Minabe, T.; Fukuda, T.; Arai, E.; Tamaru, J.-I.; Akagi, K.; Eguchi, H.; et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn. J. Clin. Oncol. 2018, 48, 514–521. [Google Scholar] [CrossRef]
- Hatta, N.; Takata, A.; Ishizawa, S.; Niida, Y. Family with MSH2 mutation presenting with keratoacanthoma and precancerous skin lesions. J. Dermatol. 2015, 42, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R.; Roggero, E.; Sudilovsky, E.C.; Tuthill, R.J.; Wood, G.S.; Sudilovsky, O. Alterations of mismatch repair protein expression in benign melanocytic nevi, melanocytic dysplastic nevi, and cutaneous malignant melanomas. Am. J. Dermatopathol. 2001, 23, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Perrett, C.; Harwood, C.; McGregor, J.; Warwick, J.; Cerio, R.; Karran, P. Expression of DNA mismatch repair proteins and MSH2 polymorphisms in nonmelanoma skin cancers of organ transplant recipients. Br. J. Dermatol. 2009, 162, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Eiberg, H.; Hansen, L.; Hansen, C.; Mohr, J.; Teglbjaerg, P.S.; Kjaer, K.W.; Teglbjærg, P.S. Mapping of hereditary trichilemmal cyst(TRICY1)to chromosome 3p24-p21.2 and exclusion of β-CATENIN and MLH1. Am. J. Med. Genet. Part A 2005, 133A, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Cilona, M.; Locatello, L.G.; Novelli, L.; Gallo, O. The mismatch repair system (MMR) in head and neck carcinogenesis and its role in modulating the response to immunotherapy: A critical review. Cancers 2020, 12, 3006. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Rass, K. Ultraviolet Damage, DNA Repair and Vitamin D in Nonmelanoma Skin Cancer and in Malignant Melanoma. Adv. Exp. Med. Biol. 2014, 810, 208–233. [Google Scholar] [CrossRef]
- Loureiro, J.B.; Abrantes, M.; Oliveira, P.; Saraiva, L. P53 in skin cancer: From a master player to a privileged target for prevention and therapy. Biochim. Biophys. Acta BBA Rev. Cancer 2020, 1874, 188438. [Google Scholar] [CrossRef]
- Vasan, K.; Anand, S.; Satgunaseelan, L.; Asher, R.; Low, H.; Palme, C.E.; Lee, J.H.; Clark, J.R.; Gupta, R. Mismatch repair protein loss in cutaneous head and neck squamous cell carcinoma. J. Surg. Oncol. 2020, 122, 1755–1760. [Google Scholar] [CrossRef]
- El Hassani, Y.; Beaulieu, J.-Y.; Tschanz, E.; Marcheix, P.-S. Localisation inhabituelle pulpaire d’un kyste trichilemmal proliférant. Chir. Main 2013, 32, 117–119. [Google Scholar] [CrossRef]
- Makiese, O.; Chibbaro, S.; Hamdi, S.; Mirone, G.; George, B. Huge proliferating trichilemmal tumors of the scalp: Report of six cases. Plast. Reconstr. Surg. 2010, 126, 18e–19e. [Google Scholar] [CrossRef] [PubMed]
- López-Ríos, F.; Rodríguez-Peralto, J.L.; Aguilar, A.; Hernández, L.; Gallego, M. Proliferating trichilemmal cyst with focal invasion. Am. J. Dermatopathol. 2000, 22, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Erdem, H.; Uzunlar, A.K.; Ozcelik, D.; Yildirim, U.; Sahiner, C.; Toplu, G. Posttraumatic giant proliferating trichilemmal cysts on the parietal region of the scalp. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 707–709. [Google Scholar] [CrossRef]
- Al-Shanawani, B.; Abdelhamid, M.M.; Al-Shomer, F.M. Giant proliferating trichilemmal tumor. Arch. Plast. Surg. 2013, 40, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Soua, Y. Giant proliferating trichilemmal cyst. Pan Afr. Med. J. 2014, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Challita, R.; Halabi, S. Giant aggressive forehead tumor: A 15-year follow-up. Clin. Pract. 2019, 9, 1172. [Google Scholar] [CrossRef]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, H.; Qiao, J.; Zhang, S.; Liu, S.; Li, N.; Lei, X.; Ning, L.; Cao, Y.; Duan, E. Expansion of hair follicle stem cells sticking to isolated sebaceous glands to generate in vivo epidermal structures. Cell Transplant. 2016, 25, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, F.; Ito, M.; Nakamura, A.; Sato, Y. Proliferating trichilemmal cyst with apocrine-acrosyringeal and sebaceous differentiation. J. Cutan. Pathol. 1991, 18, 137–141. [Google Scholar] [CrossRef]
- Dekio, S.; Imaoka, C.; Jidoi, J. Proliferating trichilemmal tumor with apocrine sweat glands. J. Dermatol. 1990, 17, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, S.F.; Aickara, D.; Price, A.; Pavlis, J.; Elgart, G.; Cho-Vega, J.H.; Wei, E.X. Giant proliferating trichilemmal cyst arising from a nevus sebaceus growing for 30 years. J. Cutan. Pathol. 2017, 44, 639–642. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, S.; Huang, X.; Chen, K.; Shen, J.; Wang, Z. Involvement of p53 mutation and mismatch repair proteins dysregulation in NNK-induced malignant transformation of human bronchial epithelial cells. BioMed Res. Int. 2014, 2014, 920275. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Ramamurthi, K.; Mishima, M.; Kondo, A.; Christen, R.D.; Howell, S.B. P53 modulates the effect of loss of DNA mis-match repair on the sensitivity of human colon cancer cells to the cytotoxic and and mutagenic effects of cisplatin. Cancer Res. 2001, 61, 1508–1516. [Google Scholar]
- Liang, S.B.; Furihata, M.; Takeuchi, T.; Sonobe, H.; Ohtsuki, Y. Reduced human mismatch repair protein expression in the development of precancerous skin lesions to squamous cell carcinoma. Virchows Arch. 2001, 439, 622–627. [Google Scholar] [CrossRef]
- Ciavattini, A.; Piccioni, M.; Tranquilli, A.L.; Filosa, A.; Pieramici, T.; Goteri, G. Immunohistochemical expression of DNA mismatch repair (MMR) system proteins (hMLH1, hMSH2) in cervical preinvasive and invasive lesions. Pathol. Res. Pract. 2005, 201, 21–25. [Google Scholar] [CrossRef]
- Young, L.; Listgarten, J.; Trotter, M.; Andrew, S.; Tron, V.A. Evidence that dysregulated DNA mismatch repair characterizes human nonmelanoma skin cancer. Br. J. Dermatol. 2007, 158, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Grayson, W.; Redston, M.; Diwan, A.H.; Warneke, C.L.; McKee, P.H.; Lev, D.; Lyle, S.; Calonje, E.; Lazar, A.J.F. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am. J. Surg. Pathol. 2008, 32, 936–942. [Google Scholar] [CrossRef]
- North, J.P.; Golovato, J.; Vaske, C.J.; Sanborn, J.Z.; Nguyen, A.; Wu, W.; Goode, B.; Stevers, M.; McMullen, K.; White, B.E.P.; et al. Cell of origin and mutation pattern define three clinically distinct classes of sebaceous carcinoma. Nat. Commun. 2018, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Macedo, S.; Fernandes, M.S.; Pestana, A.; Pardal, J.; Batista, R.; Vinagre, J.; Sanches, A.; Baptista, A.; Lopes, J.M.; et al. Prognostic significance of RAS mutations and P53 expression in cutaneous squamous cell carcinomas. Genes 2020, 11, 751. [Google Scholar] [CrossRef]
- Hwang, L.-A.; Phang, B.H.; Liew, O.W.; Iqbal, J.; Koh, X.H.; Koh, X.Y.; Othman, R.; Xue, Y.; Richards, A.M.; Lane, D.P.; et al. Monoclonal antibodies against specific p53 hotspot mutants as potential tools for precision medicine. Cell Rep. 2018, 22, 299–312. [Google Scholar] [CrossRef]
- Shi, Y.; Norberg, E.; Vakifahmetoglu-Norberg, H. Mutant p53 as a regulator and target of autophagy. Front. Oncol. 2021, 10, 607149. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, L.; Martens, M.C.; Emmert, S. Molecular biology of basal and squamous cell carcinomas. Adv. Exp. Med. Biol. 2020, 1268, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Shafi, R.; Nelson, J.; Cantrell, W.; Subhadarshani, S.; Andea, A.; Athar, M.; Elmets, C. The clinical course of actinic keratosis correlates with underlying molecular mechanisms. Br. J. Dermatol. 2019, 182, 995–1002. [Google Scholar] [CrossRef]
- Javor, S.; Gasparini, G.; Biatta, C.M.; Cozzani, E.; Cabiddu, F.; Ravetti, J.L.; Vellone, V.G.; Parodi, A. P53 staining index and zonal staining patterns in actinic keratoses. Arch. Dermatol. Res. 2021, 313, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Reichrath, S.; Vogt, T.; Römer, K. Crosstalk between vitamin D and p53 signaling in cancer: An update. Adv. Exp. Med. Biol. 2020, 1268, 307–318. [Google Scholar] [CrossRef]
- Berman, B.; Cockerell, C.J. Pathobiology of actinic keratosis: Ultraviolet-dependent keratinocyte proliferation. J. Am. Acad. Dermatol. 2013, 68, S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Murnyák, B.; Hortobágyi, T. Immunohistochemical correlates of TP53 somatic mutations in cancer. Oncotarget 2016, 7, 64910–64920. [Google Scholar] [CrossRef]
- Niyaz, M.; Ainiwaer, J.; Abudureheman, A.; Zhang, L.; Sheyhidin, I.; Turhong, A.; Cai, R.; Hou, Z.; Awut, E. Association between TP53 gene deletion and protein expression in esophageal squamous cell carcinoma and its prognostic significance. Oncol. Lett. 2020, 20, 1855–1865. [Google Scholar] [CrossRef]
- McGraw, K.L.; Nguyen, J.; Komrokji, R.S.; Sallman, D.; Al Ali, N.H.; Padron, E.; Lancet, J.E.; Moscinski, L.C.; List, A.F.; Zhang, L. Immunohistochemical pattern of p53 is a measure of TP53 mutation burden and adverse clinical outcome in myelodysplastic syndromes and secondary acute myeloid leukemia. Haematologica 2016, 101, e320–e323. [Google Scholar] [CrossRef]
- Chang, H.; Yeung, J.; Qi, C.; Xu, W. Aberrant nuclear p53 protein expression detected by immunohistochemistry is associated with hemizygous P53 deletion and poor survival for multiple myeloma. Br. J. Haematol. 2007, 138, 324–329. [Google Scholar] [CrossRef]
- Chen, M.-H.; Qi, C.X.; Saha, M.N.; Chang, H. p53 Nuclear Expression Correlates with Hemizygous TP53 Deletion and Predicts an Adverse Outcome for Patients with Relapsed/Refractory Multiple Myeloma Treated with Lenalidomide. Am. J. Clin. Pathol. 2012, 137, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Flynt, E.; Bisht, K.; Sridharan, V.; Ortiz, M.; Towfic, F.; Thakurta, A. Prognosis, Biology, and Targeting of TP53 Dysregulation in Multiple Myeloma. Cells 2020, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Zetner, D.B.; Bisgaard, M.L. Familial Colorectal Cancer Type X. Curr. Genom. 2017, 18, 341–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
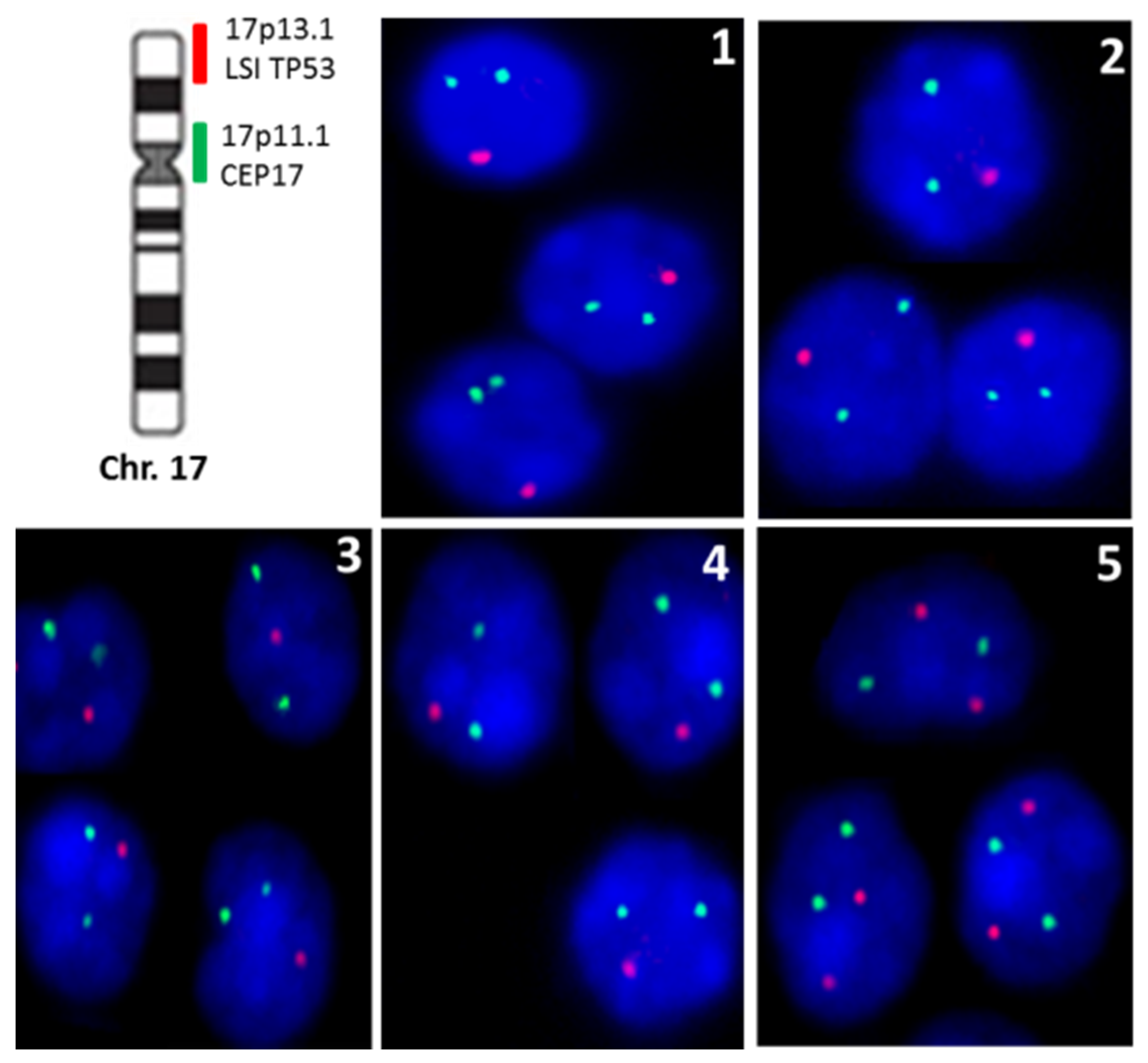
| Case | Gender | Age | Topography | Size |
|---|---|---|---|---|
| Case 1 | Female | 88 | Scalp (occipital) | 9 cm |
| Case 2 | Female | 45 | Back | 1 cm |
| Case 3 | Female | 84 | Scalp (frontal) | 3 cm |
| Case 4 | Female | 63 | Scalp (occipital) | 3 cm |
| Case 5 | Female | 69 | Scalp (parietal) | 1.5 cm |
| Case | MSH2 | MSH6 | MLH1 | PMS2 | P53 IHQ | TP53 Loss |
|---|---|---|---|---|---|---|
| Case 1 | 80% | 80% | 60% | 70% | Weak | 63% |
| Case 2 | 60% | 60% | 40% | 30% | Negative | 65% |
| Case 3 | 90% | 80% | 80% | 80% | Negative | 52% |
| Case 4 | 90% | 90% | 90% | 90% | Weak | 80% |
| Case 5 | 90% | 90% | 80% | 70% | Negative | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Sanz, R.; Sayagués, J.M.; García-Cano, P.; Azcue-Mayorga, M.; Parra-Pérez, M.d.C.; Pacios-Pacios, M.Á.; Piqué-Durán, E.; Feito, J. TP53 Abnormalities and MMR Preservation in 5 Cases of Proliferating Trichilemmal Tumours. Dermatopathology 2021, 8, 147-158. https://doi.org/10.3390/dermatopathology8020021
Martín-Sanz R, Sayagués JM, García-Cano P, Azcue-Mayorga M, Parra-Pérez MdC, Pacios-Pacios MÁ, Piqué-Durán E, Feito J. TP53 Abnormalities and MMR Preservation in 5 Cases of Proliferating Trichilemmal Tumours. Dermatopathology. 2021; 8(2):147-158. https://doi.org/10.3390/dermatopathology8020021
Chicago/Turabian StyleMartín-Sanz, Raquel, José María Sayagués, Pilar García-Cano, Mikel Azcue-Mayorga, María del Carmen Parra-Pérez, María Ángeles Pacios-Pacios, Enric Piqué-Durán, and Jorge Feito. 2021. "TP53 Abnormalities and MMR Preservation in 5 Cases of Proliferating Trichilemmal Tumours" Dermatopathology 8, no. 2: 147-158. https://doi.org/10.3390/dermatopathology8020021
APA StyleMartín-Sanz, R., Sayagués, J. M., García-Cano, P., Azcue-Mayorga, M., Parra-Pérez, M. d. C., Pacios-Pacios, M. Á., Piqué-Durán, E., & Feito, J. (2021). TP53 Abnormalities and MMR Preservation in 5 Cases of Proliferating Trichilemmal Tumours. Dermatopathology, 8(2), 147-158. https://doi.org/10.3390/dermatopathology8020021






