Comparative Clinicopathological Analysis of Oral Focal Mucinosis and Solitary Cutaneous Focal Mucinosis: A Case Series and Literature-Based Analysis
Abstract
1. Introduction
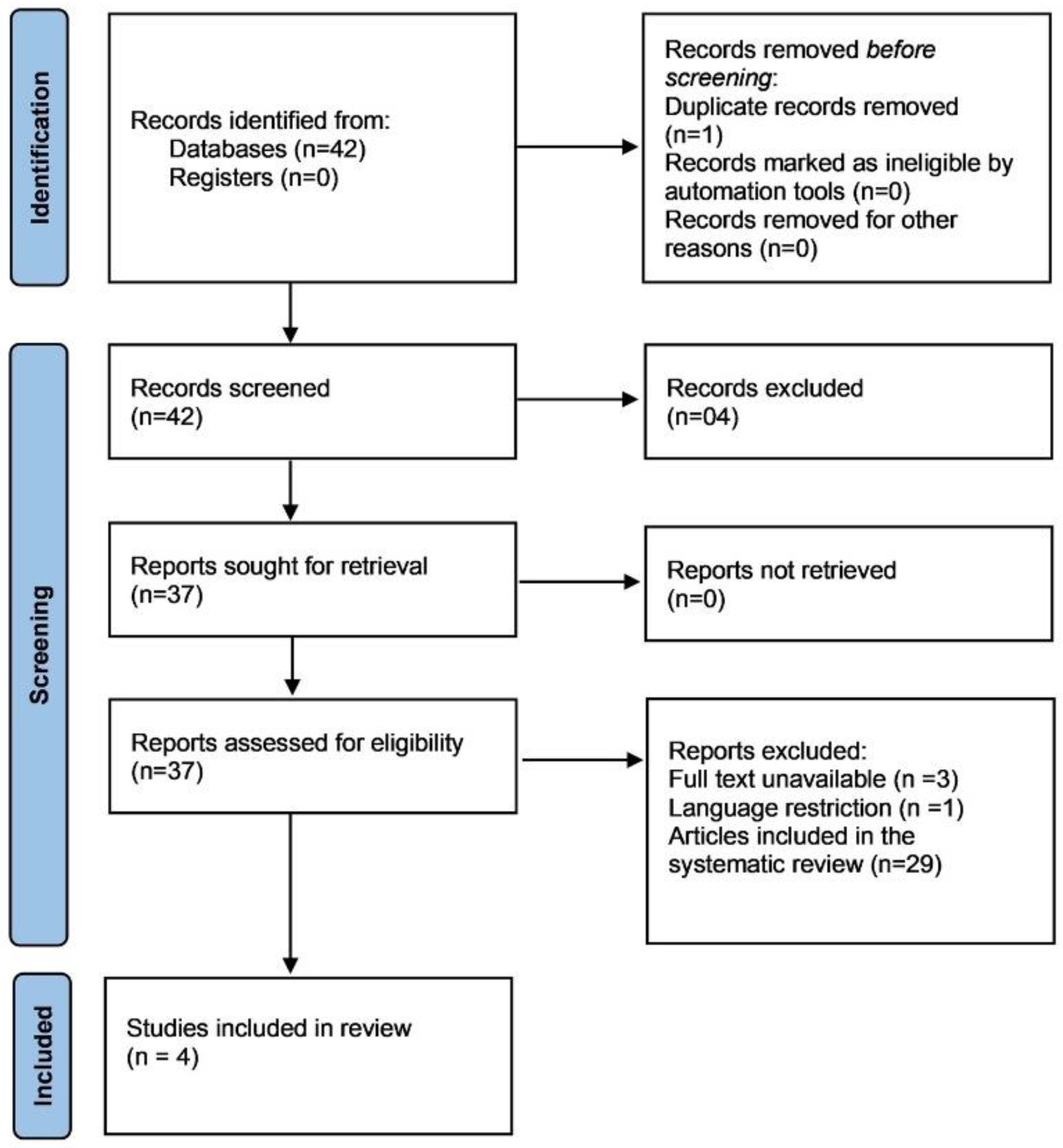
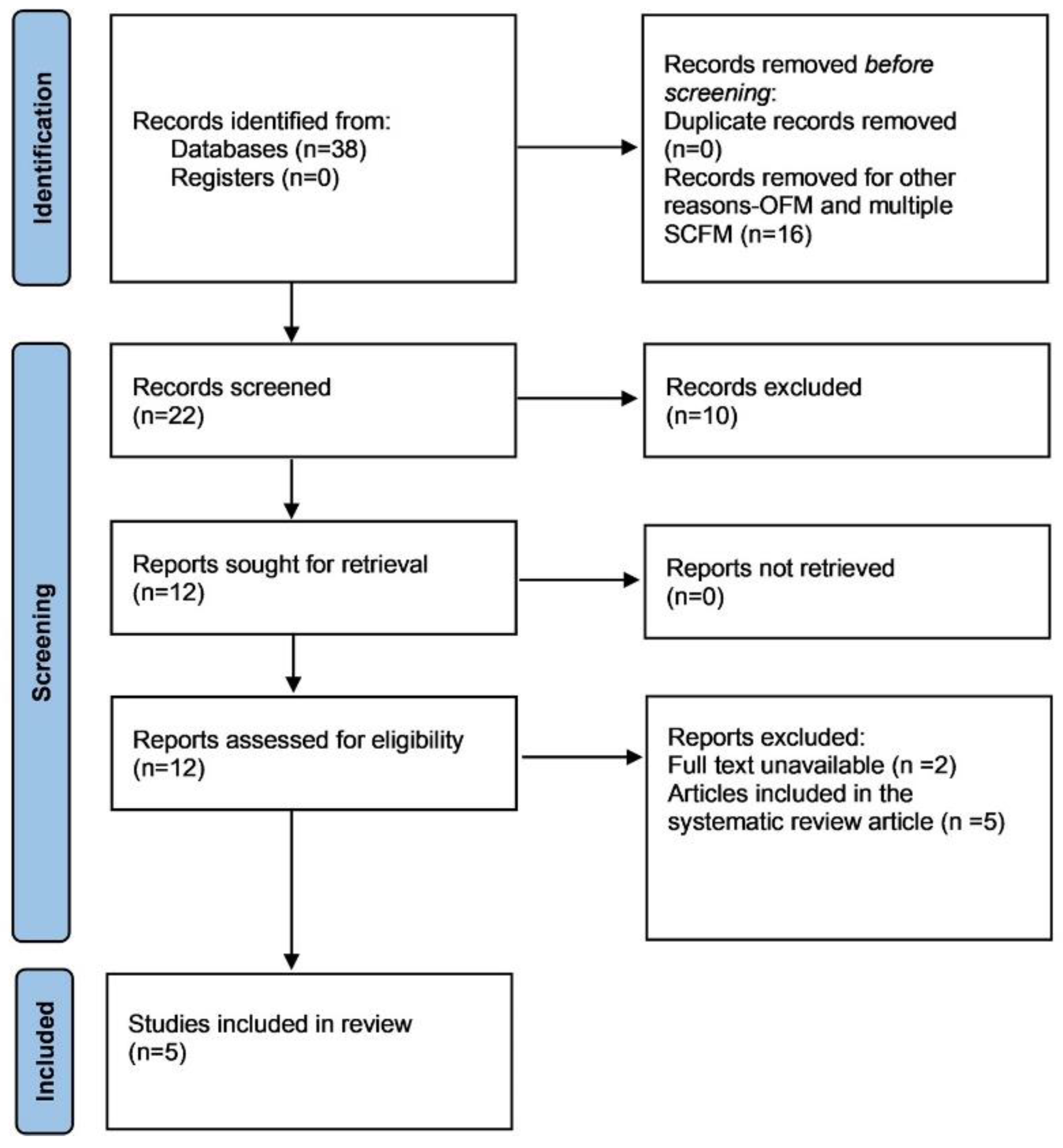
2. Materials and Methods
2.1. Case Series
2.2. Study Selection and Data Extraction from the Literature
2.2.1. OMF
2.2.2. SCFM
2.3. Statistical Analysis
3. Results
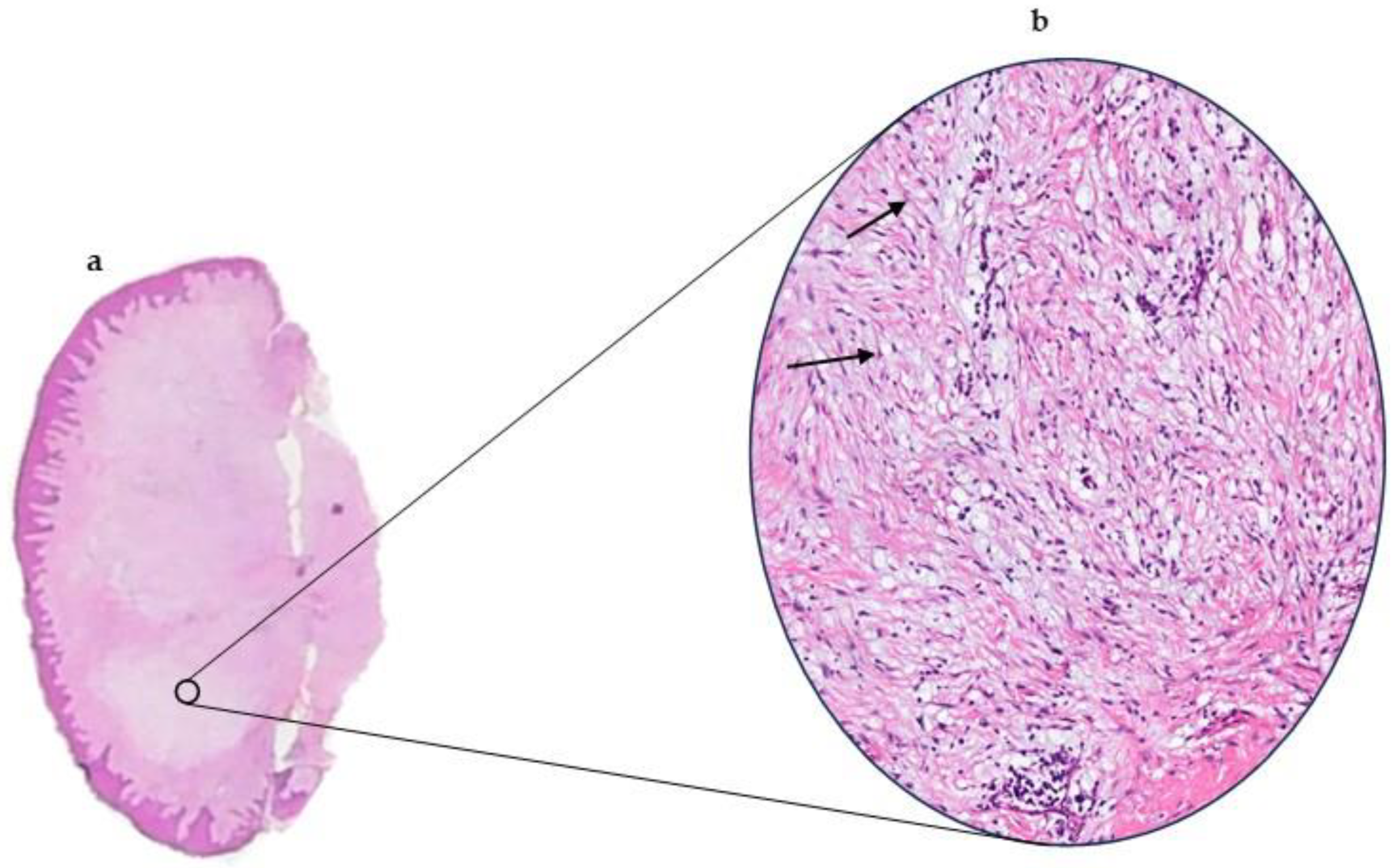
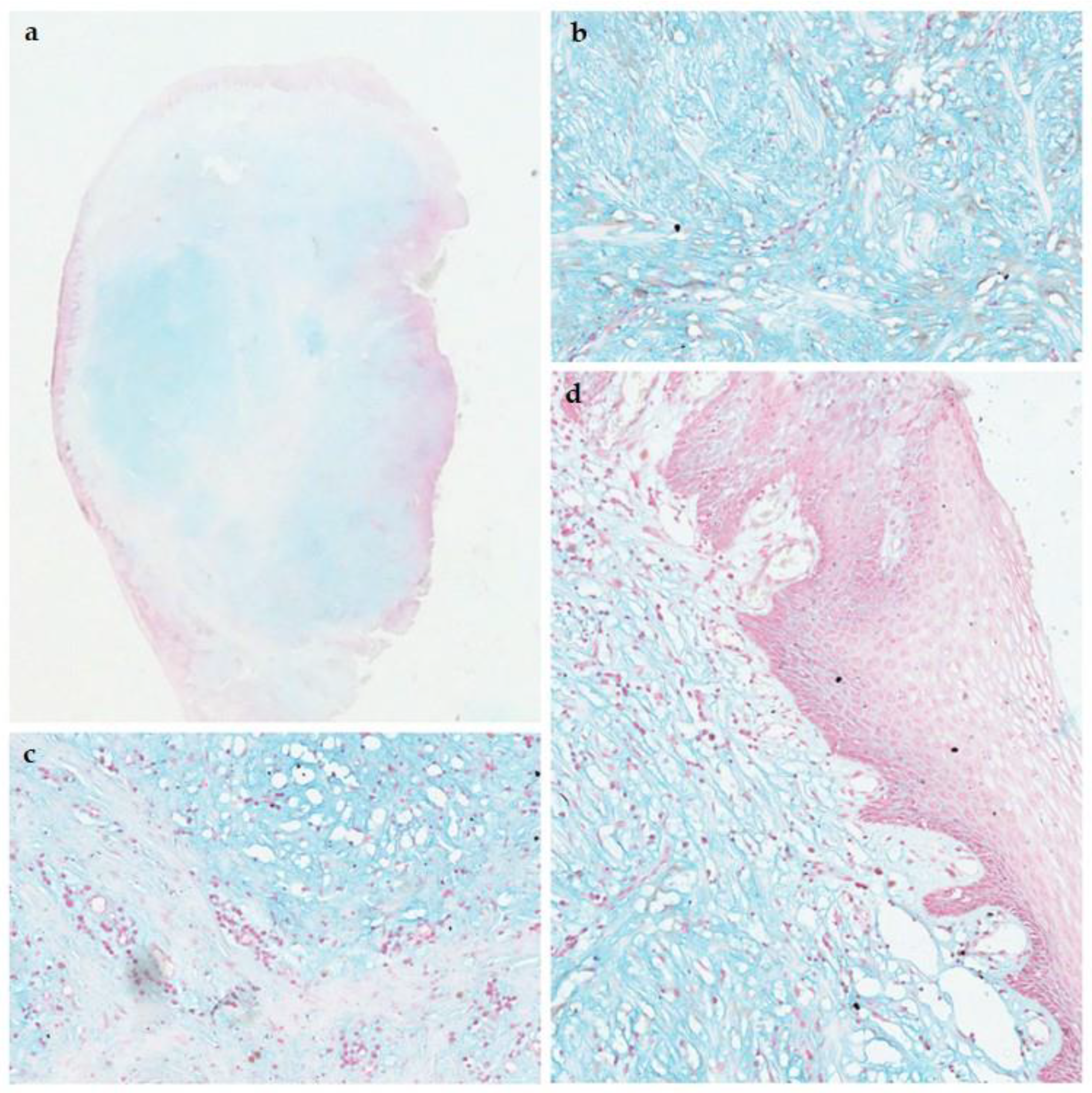
3.1. Pathological Features
3.2. Comparison with Solitary Cutaneous Focal Mucinosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OFM | Oral focal mucinosis |
| SCFM | Solitary cutaneous focal mucinosis |
References
- Cohen, P.R.; Erickson, C.P.; Calame, A. Case report and review of solitary cutaneous focal mucinosis: A unique primary cutaneous mucinosis unrelated to mucinosis-associated systemic diseases. Dermatol. Online J. 2020, 26. [Google Scholar] [CrossRef]
- Cunha, J.L.S.; Leite, A.A.; Abrantes, T.d.C.; Vervloet, L.P.; Morais, T.M.d.L.; Neto, G.d.O.P.; Kimura, T.N.L.; Ferreira, S.M.S.; de Albuquerque-Júnior, R.L.C.; Abrahão, A.C.; et al. Oral focal mucinosis: A multi-institutional study and literature review. J. Cutan. Pathol. 2020, 48, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.; Lee, L.; Kuo, T. Solitary cutaneous focal mucinosis: A clinicopathological study of 11 cases of soft fibroma-like cutaneous mucinous lesions. J. Dermatol. 2016, 44, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Tomich, C.E. Oral focal mucinosis. Oral Surg. Oral Med. Oral Pathol. 1974, 38, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, L.D.; Kirschnick, L.B.; Gabriel, A.d.F.; Silveira, F.M.; Lopes, M.A.; Wagner, V.P.; Schuch, L.F.; Martins, M.D. Oral focal mucinosis: A systematic review. Br. J. Oral Maxillofac. Surg. 2024, 62, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Krishanappa, S.J.; Channabasaviah, G.H.; Kamat, M.S.; Prakash, S.G. Oral Focal Mucinosis. Int. J. Infect. Dis. 2025, 28, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chatterjee, A.; Chatterjee, R.P.; Sinha, S.; Sultana, M.; Shah, N.; Pal, M.; Mahmud, S.A. Myxomatous Mysteries: Decoding the Oral Focal Mucinosis. Cureus 2024, 16, e67921. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Hirai, H.; Maruyama, S.; Tanuma, J.-I.; Tomihara, K. Oral Focal Mucinosis of the Tongue: Case Report and Review of the Literature. Cureus 2024, 16, e67882. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, N.; Erickson, C.; Calame, A.; Cohen, P.R. Solitary Cutaneous Focal Mucinosis. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Khopkar, U.; Garg, G.; Gutte, R.; Kharkar, V. Asymptomatic nodule over the shin. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 123. [Google Scholar] [CrossRef] [PubMed]
- Daroach, M.; Chauhan, P.; Dhiman, A. Dermoscopy of Solitary Cutaneous Focal Mucinosis. Indian Dermatol. Online J. 2024, 15, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Ortube, M.C.; Dipple, K.; Setoguchi, Y.; Kawamoto, H.K.J.D.; Demer, J.L. Ocular Manifestations of Oblique Facial Clefts. J. Craniofacial Surg. 2010, 21, 1630–1631. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, A.; de Oliveira, D.; Lopes, M.; Filho, T.; Queiroz, L.; da Silveira, E. Clinicopathological study of oral focal mucinosis: A retrospective case series. Med. Oral Patol. Oral Y Cirugia Bucal 2018, 23, E401–E405. [Google Scholar] [CrossRef] [PubMed]
- van Roggen, G.; Hogendoorn, P.C.; Fletcher, M. Myxoid tumours of soft tissue. Histopathology 1999, 35, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Holloway, P.; Kay, E.; Leader, M. Myxoid tumours: A guide to the morphological and immunohistochemical assessment of soft tissue myxoid lesions encountered in general surgical pathology. Curr. Diagn. Pathol. 2005, 11, 411–425. [Google Scholar] [CrossRef]
- Biondo, G.; Sola, S.; Pastorino, C.; Massone, C. Clinical, dermoscopic, and histologic aspects of two cases of cutaneous focal mucinosis. An. Bras. de Dermatol. 2019, 94, 334–336. [Google Scholar] [CrossRef]
- Nebrida, M.L.A.; Tay, Y. Cutaneous Focal Mucinosis: A Case Report. Pediatr. Dermatol. 2002, 19, 33–35. [Google Scholar] [CrossRef]
| Case | Sex/Age | Location | Duration (Months) | Size (cm) | Symptoms | Clinical impression | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | M/31 | Palate | 12 | 4 | Asymptomatic | Pleomorphic adenoma | Ex |
| 2 | F/70 | Gingiva | NA | NA | Asymptomatic | NA | Ex |
| 3 | F/33 | Gingiva | 12 | 2 | Asymptomatic | Suspicious lesion | Ex |
| 4 | M/14 | Gingiva | 3 | 2 | Asymptomatic | Suspicious lesion | Ex |
| 5 | F/44 | Gingiva | 3 | 1 | Bleeding tendency | Fibrous epulis | Ex |
| 6 | M/54 | Palate | NA | NA | Asymptomatic | Traumatic lesion | In |
| 7 | F/NA | Gingiva | 12 | 1.5 | Bleeding on brushing | Epulis | Ex |
| 8 | F/38 | Gingiva | 2 | 3 | Asymptomatic | Fibroepithelial polyp | In |
| 9 | F/21 | Gingiva | NA | 1 | Asymptomatic | Osteoma | Ex |
| 10 | F/57 | Alveolar ridge mucosa | 4 | 2 | Asymptomatic | Fibrous epulis | Ex |
| 11 | M/33 | Palate | 36 | 2 | Asymptomatic | Fibroepithelial polyp | Ex |
| 12 | F/59 | Gingiva | 3 | 2 | Asymptomatic | NA | Ex |
| 13 | M/48 | Buccal sulcus | 2.5 | 5 | Asymptomatic | Giant cell tumour | Ex |
| 14 | F/59 | Gingiva | 4 | 1 | Asymptomatic | Fibrous epulis | In |
| 15 | F/49 | Alveolar ridge mucosa | NA | NA | Asymptomatic | NA | Ex |
| 16 | M/48 | Palate | 12 | 3 | Asymptomatic | NA | Ex |
| 17 | F/60 | Alveolar ridge mucosa | 2.5 | 3 | Asymptomatic | Fibroepithelial polyp | Ex |
| 18 | F/28 | Palate | NA | 0.3 | Asymptomatic | Fibroepithelial polyp | Ex |
| 19 | M/58 | Alveolar ridge mucosa | 3 | 2.5 | Asymptomatic | Recurrent - Oral focal mucinosis | Ex |
| 20 | M/58 | Gingiva | NA | 3 | Asymptomatic | Gingival epulis | Ex |
| 21 | M/NA | Palate | 36 | NA | Asymptomatic | Papilloma | Ex |
| 22 | M/48 | Gingiva | NA | 1 | Easily bleeding | Granuloma | Ex |
| 23 | F/14 | Gingiva | 0.5 | 1 | Asymptomatic | Pyogenic granuloma | Ex |
| 24 | M/44 | Alveolar ridge mucosa | 6 | 2 | Asymptomatic | Pyogenic granuloma | Ex |
| 25 | F/48 | Gingiva | NA | NA | Asymptomatic | Giant cell granuloma | Ex |
| 26 | M/65 | Gingiva | 12 | 3 | Asymptomatic | NA | NA |
| 27 | F/36 | Gingiva | 7 | 4 | Asymptomatic | Fibrous epulis | Ex |
| 28 | M/56 | Alveolar ridge mucosa | 12 | 0.8 | Asymptomatic | Fibrous epulis | Ex |
| 29 | F/19 | Gingiva | 24 | 0.5 | Asymptomatic | Fibroepithelial polyp | Ex |
| 30 | F/40 | Alveolar ridge mucosa | 3.5 | 0.5 | Asymptomatic | Fibroepithelial polyp | Ex |
| 31 | F/62 | Gingiva | 2 | NA | Asymptomatic | Odontogenic fibroma | Ex |
| 32 | M/36 | Gingiva | 144 | 3 | Asymptomatic | Fibroepithelial polyp | Ex |
| 33 | M/64 | Retromolar region | NA | NA | Asymptomatic | NA | In |
| 34 | M/21 | Gingiva | 8 | NA | Asymptomatic | Fibroepithelial polyp | NA |
| 35 | F/51 | Palate | 1 | 1 | Asymptomatic | Fibroepithelial polyp | Ex |
| 36 | M/15 | Gingiva | NA | 2 | Asymptomatic | Non-specific localized gingival hypertrophy | NA |
| 37 | M/NA | Gingiva | 42 | 1.5 | Asymptomatic | Gingival polyp | NA |
| 38 | F/53 | Gingiva | 0.5 | 6 | Asymptomatic | Malignant lesion | NA |
| 39 | M/45 | Alveolar ridge mucosa | NA | NA | Asymptomatic | NA | Ex |
| Variable | Present Series | Literature Review |
|---|---|---|
| Sex | n = 39 | n = 116 |
| Female | 20 (51.28%) | 83 (71.55%) |
| Male | 19 (48.71%) | 33 (28.45%) |
| Ratio | 1.05: 1 | 2.52: 1 |
| Age (years) | n = 36 | n = 113 |
| Mean (SD) | 43.86 (15.80) | 40.74 (17.79) |
| Range | 14–70 | 2–88 |
| Not informed | 3 | |
| Anatomical location | n = 39 | n = 114 |
| Gingiva | 22 (56.41%) | 59 (51.75%) |
| Palate | 7 (17.95%) | 19 (16.67%) |
| Alveolar ridge mucosa | 8 (20.51%) | 13 (11.40%) |
| Buccal mucosa | 1 (2.56%) | 4 (3.51%) |
| Retromolar region | 1 (2.56%) | 5 (4.39%) |
| Tongue | 10 (8.77%) | |
| Lip | 3 (2.63%) | |
| Floor of the mouth | 1 (0.88%) | |
| Recurrence | n = 39 | n = 69 |
| Yes | 1 (2.56%) | 1 (1.45%) |
| No | 38 (97.44%) | 68 (98.55%) |
| Lesion size (cm) | n = 30 | n = 86 |
| Mean (SD) | 2.15 (1.36) | 1.37 (1.41) |
| Range | 0.3–6 | 0.1–10 |
| Duration of the lesion (months) | n = 28 | n = 72 |
| Mean (SD) | 21.25 (27.75) | 17.55 (23.83) |
| Range | 0.5–144 | 1–120 |
| Management | n = 34 | n = 104 |
| Surgical excision | 30 (88.24%) | 98 (94.23%) |
| Incisional biopsy | 4 (11.76%) | 6 (5.77%) |
| Clinical hypothesis | n = 31 | n = 101 |
| Reactive lesions | 23 (74.19%) | 99 (98.02%) |
| Benign tumours | 5 (16.13%) | |
| Malignant lesions | 3 (9.68%) | 2 (1.98%) |
| Solitary Cutaneous Focal Mucinosis | Oral Focal Mucinosis | |
|---|---|---|
| Cases | 138 | 155 |
| Men/women | 1: 0.6 | 0.5: 1 |
| Mean age (years) | 52 | 41 |
| Age range (years) | 6–86 | 2–88 |
| Duration of lesion | Months-years | 18.58 months |
| Number of lesions | 1 | 1 |
| Symptoms | Asymptomatic = 138 (100%) | Asymptomatic = 114 (89.76%) Symptomatic = 13 (10.24%) |
| Size (mm) | 2–20 | 1–100 |
| Morphology | Nodule Papule Mass | Swelling Nodule Lump |
| Site | Extremities = 76 (55.07%) Trunk = 44 (31.88%) Head and neck = 18 (13.04%) | Gingiva = 81 (52.94%) Palate = 26 (16.99%) Alveolar ridge mucosa = 21 (13.73%) Buccal mucosa = 5 (3.27%) Retromolar region = 6 (3.92%) Tongue = 10 (6.54%) Lip = 3 (1.96%) Floor of the mouth = 1 (0.65%) |
| Treatment | Excision = 26 Other (biopsy/curettage/electrodessication and curettage) = 3 | Excision = 128 (89.51%) Other (incisional biopsy) = 10 (6.99%) |
| Recurrence | 0% | 1.85% |
| Feature | SCFM | OFM |
|---|---|---|
| Architecture | Well-circumscribed, non-encapsulated dermal lesion. | Well-circumscribed, non-encapsulated lesion in the lamina propria. |
| Stroma | Mucin-rich (myxoid) dermis. | Abundant myxoid (mucin-rich) connective tissue. |
| Cells | Scattered spindle cells; no fascicular or storiform pattern. | Scattered spindle-shaped fibroblasts; lacks fascicular or storiform arrangement. |
| Vascularity | Usually not prominent; differs from superficial angiomyxoma. | Generally sparse, without prominent vasculature. |
| Inflammation | Minimal or absent inflammatory infiltrate; neutrophils rare. | Minimal or absent inflammatory infiltrate. |
| Special stains | Positive for Alcian Blue. | Positive for Alcian Blue (acidic mucopolysaccharides). |
| Immunohistochemistry | SMA-negative; CD34-negative; CD68-negative. | Typically SMA-negative; CD34-negative; CD68-negative. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickramasinghe, W.M.S.N.; Jayasooriya, P.R.; Mendis, B.R.R.N.; Lombardi, T. Comparative Clinicopathological Analysis of Oral Focal Mucinosis and Solitary Cutaneous Focal Mucinosis: A Case Series and Literature-Based Analysis. Dermatopathology 2025, 12, 38. https://doi.org/10.3390/dermatopathology12040038
Wickramasinghe WMSN, Jayasooriya PR, Mendis BRRN, Lombardi T. Comparative Clinicopathological Analysis of Oral Focal Mucinosis and Solitary Cutaneous Focal Mucinosis: A Case Series and Literature-Based Analysis. Dermatopathology. 2025; 12(4):38. https://doi.org/10.3390/dermatopathology12040038
Chicago/Turabian StyleWickramasinghe, Wickramasinghe Mudiyanselage Sithma Nilochana, Primali Rukmal Jayasooriya, Balapuwaduge Ranjit Rigobert Nihal Mendis, and Tommaso Lombardi. 2025. "Comparative Clinicopathological Analysis of Oral Focal Mucinosis and Solitary Cutaneous Focal Mucinosis: A Case Series and Literature-Based Analysis" Dermatopathology 12, no. 4: 38. https://doi.org/10.3390/dermatopathology12040038
APA StyleWickramasinghe, W. M. S. N., Jayasooriya, P. R., Mendis, B. R. R. N., & Lombardi, T. (2025). Comparative Clinicopathological Analysis of Oral Focal Mucinosis and Solitary Cutaneous Focal Mucinosis: A Case Series and Literature-Based Analysis. Dermatopathology, 12(4), 38. https://doi.org/10.3390/dermatopathology12040038







