Abstract
Dermatofibroma is a common mesenchymal skin lesion that typically presents as a firm, slow-growing nodule. Generally, such lesions are asymptomatic; however, they can also cause discomfort in some cases. Ulceration is an uncommon feature of dermatofibroma, and diagnosis in such cases is often difficult. We report a case of a 67-year-old female with multiple comorbidities, including pancreatic cancer undergoing neoadjuvant chemotherapy, who was admitted for acute pulmonary embolism. The patient presented with an incidental medial thigh lesion. The lesion was asymptomatic, ulcerated, and oozing pus one month before presentation. Clinical examination revealed a 3 × 2 cm deep ulcer with a punched-out edge, a dry yellow-white base, and a firm violaceous border. Histopathology confirmed dermatofibroma with epidermal hyperplasia, dermal spindle cell proliferation, histiocytes, and collagen trapping. Immunohistochemistry was positive for CD68, CD10, and Factor XIII. Due to the deteriorating condition of the patient, no intervention was provided to her, and she succumbed to her primary illness. This case is unique due to its atypical ulcerative presentation in a patient with complex systemic illness and emphasizes distinguishing between benign lesions and malignant mimics, especially in cases which have ambiguous clinical presentation.
1. Introduction
Dermatofibroma, also known as benign fibrous histiocytoma, is a common benign skin tumor characterized by slow-growing, firm, well-circumscribed nodules. These lesions are often asymptomatic but may occasionally present with pruritus or tenderness [1,2,3]. Dermatofibromas predominantly affect adult women and are frequently located on the lower extremities [4]. Dermatofibromas account for approximately 3% of all excised cutaneous lesions [2]. However, this figure may underestimate the true prevalence, as many lesions remain clinically silent and are not subjected to histopathological examination.
The etiology of dermatofibroma is not fully understood. Local trauma, such as insect bites or surgical scars, is a proposed trigger [5], though reported in only a minority of cases [6]. Immunologic responses, particularly in immunocompromised patients, have also been suggested [7]. Dermatofibromas are typically less than 1 cm but can exceed 3 cm, with rare giant variants over 5 cm. They often exhibit a “dimple sign” upon lateral compression due to attachment to underlying tissue [8]. While they can occur anywhere, the extremities are the most common site, with or without skin discoloration [9]. Recognized variants include cellular, aneurysmal, atypical, and perforating dermatofibromas, each with distinct histological features [9]. Ulceration, as seen in our case, is a rare clinical manifestation rather than a distinct histological subtype [9,10].
Diagnosis typically depends on clinical evaluation and history. Histologically, dermatofibromas are composed of intersecting fascicles of spindle-shaped fibroblasts and histiocytes arranged in a storiform pattern embedded within a collagen-rich stroma [11]. These may be accompanied by overlying epidermal hyperplasia, acanthosis, or hyperkeratosis [12]. In deeper variants, extension into the superficial subcutis is more common [2].
In ulcerated dermatofibromas, histopathological examination is even more critical. Ulceration leads to the disruption of the epidermis and superficial dermis, often accompanied by a reactive inflammatory infiltrate composed of lymphocytes, plasma cells, and neutrophils [13]. Thick sclerotic bundles of collagen fibers interwoven with spindle cells are typical, with peripheral collagen trapping considered a supportive diagnostic feature [14]. Ulceration may also cause necrosis or hemorrhage, complicating interpretation and requiring careful tissue sampling [1].
A panel of immunohistochemical stains is often necessary to rule out malignant mimics. Dermatofibromas usually express Factor XIIIa and are negative for CD34 [15], which helps differentiate them from dermatofibrosarcoma protuberans (CD34-positive and Factor XIIIa-negative) [10]. The gradual histological transition from lesional to normal dermis and the infiltration of spindle cells into surrounding collagen are also distinguishing features [16,17].
This report presents a rare, ulcerated dermatofibroma in an immunocompromised patient, mimicking more aggressive neoplasms [18]. By integrating clinical, histopathological, and immunohistochemical findings, we aim to highlight diagnostic challenges and enhance awareness of this uncommon presentation.
2. Case Presentation
A 67-year-old female with hypertension, diabetes mellitus, hypothyroidism, and pancreatic cancer on neoadjuvant chemotherapy was admitted for acute pulmonary embolism. During hospitalization, a dermatology consultation was requested for an incidental lesion on the medial thigh. The patient reported a small, asymptomatic nodule appearing three months prior, which enlarged, ulcerated, and began oozing pus one month before evaluation.
Clinical examination revealed a non-tender, oval-shaped, 3 × 2 cm deep ulcer with a punched-out edge, yellow-white dry base, and firm violaceous border (Figure 1). No lymphadenopathy was noted. Differential diagnoses included blastomycosis, ecthyma gangrenosum, and cutaneous metastasis from pancreatic cancer [11].
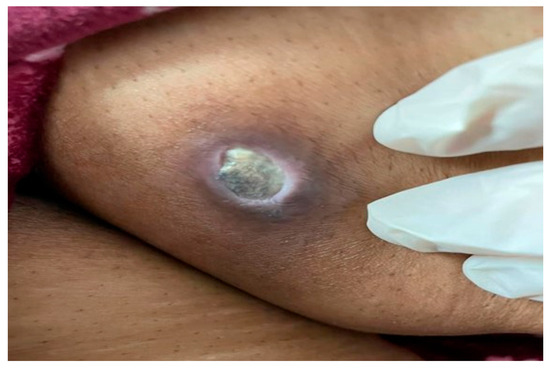
Figure 1.
Clinical image showing a non-tender, oval-shaped deep ulcer measuring 3 × 2 cm on the medial aspect of the thigh, characterized by a punched-out edge, yellowish-white dry base, and a firm violaceous border.
A 4 mm punch biopsy was chosen due to the patient’s frail condition and the need for a minimally invasive procedure to balance diagnostic yield with procedural risk. The histopathology of hematoxylin and eosin (H&E)-stained sections showed epidermal hyperplasia with focal basal layer hyperpigmentation, a non-encapsulated proliferation of spindle-shaped fibroblasts and histiocytes in a storiform pattern, and prominent collagen trapping with sclerotic bundles (Figure 2A,B). These findings confirmed dermatofibroma [19].
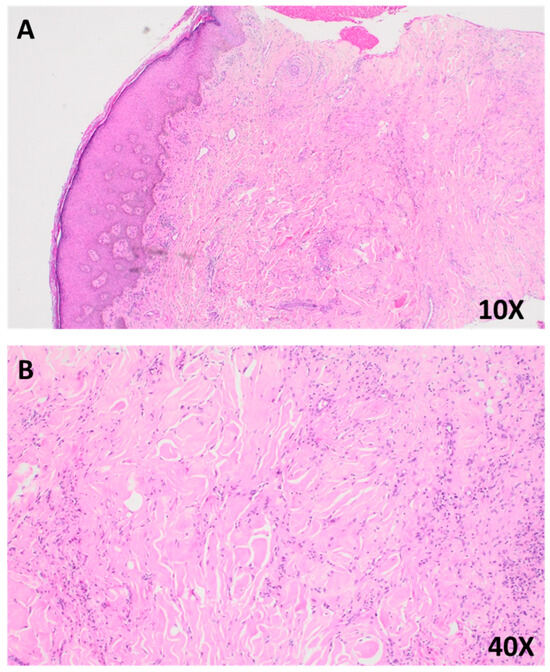
Figure 2.
(A) Photomicrograph of a hematoxylin and eosin (H&E)-stained section at 10× magnification, showing epidermal hyperplasia with basal layer hyperpigmentation and prominent collagen trapping within the dermis. (B) Higher magnification view (40×), highlighting interlacing bundles of spindle-shaped fibroblasts and histiocytes embedded within a collagen-rich stroma, consistent with features of dermatofibroma.
Immunohistochemical staining was performed to support the histopathological diagnosis and rule out malignant mimics. The lesional spindle cells exhibited strong cytoplasmic positivity for CD10 (Figure 3A) and CD68 (Figure 3C), confirming the fibrohistiocytic nature of the lesion. In addition, the cells showed diffuse positivity for Factor XIIIa, a marker characteristic of dermal dendritic cells. Notably, the lesion was negative for CD34 (Figure 3B), effectively ruling out dermatofibrosarcoma protuberans, which typically demonstrates strong CD34 positivity. This immunoprofile supports the diagnosis of dermatofibroma.
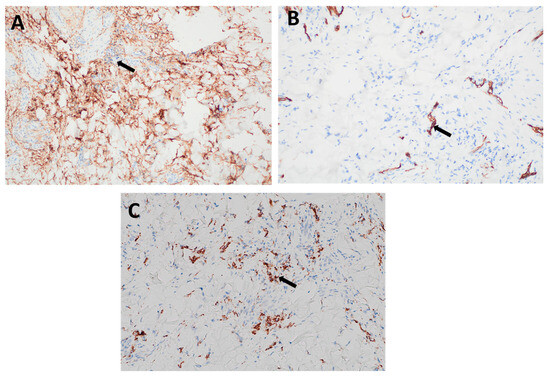
Figure 3.
Immunohistochemical staining of the lesion: (A) Strong cytoplasmic positivity for CD10 in dermal spindle cells (arrow), supporting a diagnosis of dermatofibroma. (B) Negative staining for CD34 in the lesional spindle cells (arrow), helping to differentiate the lesion from dermatofibrosarcoma protuberans, which typically shows strong CD34 positivity. (C) Positive staining for CD68 (arrow), indicating the presence of histiocytic cells, consistent with the fibrohistiocytic nature of dermatofibroma.
These histopathological and immunohistochemical findings confirmed the diagnosis of dermatofibroma. Bacterial and fungal cultures of the lesion were negative, excluding infectious etiologies. Given the patient’s advanced comorbidities and poor prognosis, no intervention was pursued for the dermatofibroma, prioritizing the management of her primary illness. The patient later succumbed to complications from pancreatic cancer.
3. Discussion
Dermatofibroma typically presents as a firm, asymptomatic nodule, but ulceration and sterile suppuration, as in this case, are rare, occurring in only 6% of cases per Kelati et al. [18]. This presentation in an immunocompromised patient with pancreatic cancer and chemotherapy adds diagnostic complexity. Similar cases in immunosuppressed patients are scarce. Beatrous et al. reported multiple dermatofibromas in patients with autoimmune diseases or HIV, often managed conservatively or with excision [11]. Lungu et al. described an ulcerated dermatofibroma in a 31-year-old male, initially mistaken for keratoacanthoma, treated with excision [11]. In contrast, our patient’s lesion was not excised due to her critical condition, as invasive procedures posed significant risks given her comorbidities and ongoing chemotherapy. Dermatofibromas are benign lesions and only rarely exhibit malignant behavior [20]. However, atypical presentations such as ulceration can clinically mimic malignant melanoma, squamous cell carcinoma, or soft tissue sarcomas [17]. Immunohistochemistry is critical for distinguishing dermatofibromas (Factor XIIIa-positive and CD34-negative) from dermatofibrosarcoma protuberans (CD34-positive and Factor XIIIa-negative) [21]. Excisional biopsy is the standard for diagnosis, with recurrence rare when margins are clear [22]. Additionally, the lesion dimensions and edge characteristics found on histopathology usually correspond closely with sonographic observations. Histologically, a combination of fibroblasts, histiocytes, and thick collagen fibers give dermatofibromas their characteristic appearance [3].
The 4 mm punch biopsy was selected to minimize procedural risks in this frail patient while providing sufficient tissue for histopathological and immunohistochemical analysis [19]. Although excisional biopsy is preferred for ulcerated lesions to ensure adequate sampling, especially when malignancy is suspected, studies suggest punch biopsies can be diagnostic for dermatofibromas when targeted appropriately [17]. In this case, the biopsy confirmed the diagnosis, supported by characteristic collagen trapping and immunohistochemistry.
The ulceration in this case likely resulted from chronic mechanical pressure on the medial thigh, which precipitated localized ischemia and tissue damage, ultimately culminating in epidermal breakdown. This primary insult was compounded by the patient’s diabetes mellitus and chemotherapy-induced vascular compromise; both systemic factors are well known to impair microcirculation and hinder wound healing processes [23,24]. Taken together, these local and systemic factors created an environment conducive to ulcer formation. This pathogenesis aligns with published reports of perforating dermatofibroma, a rare variant characterized by transepidermal elimination (epidermal breakdown) [25]. Notably, perforating dermatofibroma is characterized by lesions that breach the epidermis and ulcerate—a pattern akin to that observed in the present case [26]. Furthermore, repeated cultures of the wound exudate yielded no microbial growth, effectively ruling out an infectious etiology for the ulceration [27]. Consequently, the purulent discharge is best interpreted as sterile suppuration secondary to a robust reactive inflammatory response rather than an active infection [28].
Differential diagnoses included squamous cell carcinoma, basal cell carcinoma, amelanotic melanoma, and cutaneous metastasis. Histopathology was critical, showing epidermal hyperplasia, spindle cell proliferation, and collagen trapping [4]. Immunohistochemistry (CD68-, CD10-, and Factor XIIIa-positive; CD34-negative) distinguished dermatofibroma from dermatofibrosarcoma protuberans, which shows deeper infiltration and CD34 positivity [15,29,30]. Other differentials, like pyoderma gangrenosum or blastomycosis, were ruled out by the absence of neutrophilic infiltration or budding yeast [31]. Although benign, dermatofibromas may clinically resemble malignant melanomas or soft tissue sarcomas—especially in cases with atypical features such as ulceration or erosion [32]. Ulcerated dermatofibroma is a rare variant with damage to the epidermis and superficial dermis, often mistaken for more aggressive lesions. Diagnosis is primarily histopathological, with excisional biopsy being the standard approach; recurrence is rare when excised with intact margins [3].
Management typically involves excision for symptomatic or ambiguous lesions, with low recurrence rates [29]. Alternative treatments, like pulsed dye lasers, have shown 73% patient satisfaction in cosmetically sensitive areas [33]. In this case, the decision to forgo intervention was based on the patient’s poor prognosis and high surgical risk, prioritizing palliative care [34].
Although ultrasound and dermoscopy were not performed in this case, these tools may offer additional diagnostic value in evaluating atypical dermatofibromas. Dermoscopy often shows a central white area with a pigmented rim, while ultrasound reveals avascular lesions with spiculated borders [25,30]. Electrical impedance spectroscopy, explored by Aberg et al., showed limited diagnostic specificity for dermatofibromas [35], whereas fluorescence in situ hybridization can confirm dermatofibrosarcoma protuberans in uncertain cases [36]. These tools were unavailable in this case but could aid future diagnoses [37].
4. Conclusions
This case highlights a rare ulcerated and suppurative dermatofibroma in an immunocompromised patient, mimicking malignant or infectious conditions. Accurate diagnosis required integrating clinical, histopathological, and immunohistochemical findings. The decision to avoid intervention was justified by the patient’s critical condition. This report emphasizes considering atypical dermatofibroma variants in ulcerated lesions, particularly in vulnerable populations, and underscores the value of diagnostic tools like dermoscopy and ultrasound when available.
Author Contributions
L.A. contributed to the conceptualization, clinical assessment, and critical manuscript revision. R.A. (Renad AlKanaan) contributed to the literature review, manuscript writing, and manuscript editing. R.A. (Rema Aldihan) contributed to the clinical assessment and manuscript writing. A.A. contributed to the clinical assessment and manuscript writing. S.A. contributed to the pathological diagnosis and final review of the histological content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval is not required for this study in accordance with local or national guidelines.
Informed Consent Statement
Written informed consent was obtained from the patient.
Data Availability Statement
All data generated or analyzed during this study are presented in this article. For further information, inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- He, S.; Wen, Z. A Clinical and Histopathological Analysis of 147 Cases of Dermatofibroma (Benign Fibrous Histiocytoma). Adv. Biosci. Biotechnol. 2024, 15, 590–601. [Google Scholar] [CrossRef]
- Myers, D.J.; Fillman, E.P. Dermatofibroma. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2025. [Google Scholar]
- Gershtenson, P.C.; Krunic, A.L.; Chen, H.M. Multiple clustered dermatofibroma: Case report and review of the literature. J. Cutan. Pathol. 2010, 37, e42–e45. [Google Scholar] [CrossRef]
- Han, T.Y.; Chang, H.S.; Lee, J.H.; Lee, W.M.; Son, S.J. A clinical and histopathological study of 122 cases of dermatofibroma (benign fibrous histiocytoma). Ann. Dermatol. 2011, 23, 185–192. [Google Scholar] [CrossRef]
- Bandyopadhyay, M.R.; Besra, M.; Dutta, S.; Sarkar, S. Dermatofibroma: Atypical Presentations. Indian J. Dermatol. 2016, 61, 121. [Google Scholar] [CrossRef]
- Stivala, A.; Lombardo, G.A.; Pompili, G.; Tarico, M.S.; Fraggetta, F.; Perrotta, R.E. Dermatofibrosarcoma protuberans: Our experience of 59 cases. Oncol. Lett. 2012, 4, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Khiewplueang, K.; Thanomkitti, K. Multiple Eruptive Dermatofibromas in a Patient with Systemic Lupus Erythematosus, Lupus Nephritis and Receiving Immunosuppressive Drugs: A Case Report. Thai J. Dermatol. 2022, 38, 154–158. [Google Scholar]
- Waldman, R.A.; Grant-Kels, J.M. 10—Benign brown-black and pigmented skin growths. In Dermatology for the Primary Care Provider; Waldman, R.A., Grant-Kels, J.M., Eds.; Elsevier: Philadelphia, PA, USA, 2022; pp. 211–219. [Google Scholar]
- Hueso, L.; Sanmartín, O.; Alfaro-Rubio, A.; Serra-Guillén, C.; Martorell, A.; Llombart, B.; Requena, C.; Nagore, E.; Botella-Estrada, R.; Guillén, C. Giant dermatofibroma: Case report and review of the literature. Actas Dermosifiliogr. 2007, 98, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Yus, E.; Soria, L.; de Eusebio, E.; Requena, L. Lichenoid, erosive and ulcerated dermatofibromas. Three additional clinico-pathologic variants. J. Cutan. Pathol. 2000, 27, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Beatrous, S.V.; Riahi, R.R.; Grisoli, S.B.; Cohen, P.R. Associated conditions in patients with multiple dermatofibromas: Case reports and literature review. Dermatol. Online J. 2017, 23, 5. [Google Scholar] [CrossRef]
- Supsrisunjai, C.; Hsu, C.K.; Michael, M.; Duval, C.; Lee, J.Y.W.; Yang, H.S.; Huang, H.Y.; Chaikul, T.; Onoufriadis, A.; Steiner, R.A.; et al. Coagulation Factor XIII—A Subunit Missense Mutation in the Pathobiology of Autosomal Dominant Multiple Dermatofibromas. J. Investig. Dermatol. 2020, 140, 624–635.e627. [Google Scholar] [CrossRef]
- González-Vilas, D.; García-Gavín, J.; Ginarte, M.; Rodríguez-Blanco, I.; Toribio, J. Ulcerated dermatofibroma with osteoclast-like giant cells. J. Cutan. Pathol. 2009, 36 (Suppl. 1), 16–19. [Google Scholar] [CrossRef]
- González Vela, M.C.; Val-Bernal, J.F.; Martino, M.; Gonzalez-López, M.; García-Alberdi, E.; Hermana, S. Sclerotic fibroma-like dermatofibroma: An uncommon distinctive variant of dermatofibroma. Histol. Histopathol. 2005, 20, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Karlidag, T.; Keles, E.; Orhan, I.; Kaplama, M.E.; Cobanoglu, B. Giant ulcerative dermatofibroma. Case Rep. Otolaryngol. 2013, 2013, 254787. [Google Scholar] [CrossRef]
- Agaimy, A. The many faces of Atypical fibroxanthoma. Semin. Diagn. Pathol. 2023, 40, 306–312. [Google Scholar] [CrossRef]
- Orzan, O.A.; Dorobanțu, A.M.; Gurău, C.D.; Ali, S.; Mihai, M.M.; Popa, L.G.; Giurcăneanu, C.; Tudose, I.; Bălăceanu, B. Challenging patterns of atypical dermatofibromas and promising diagnostic tools for differential diagnosis of malignant lesions. Diagnostics 2023, 13, 671. [Google Scholar] [CrossRef] [PubMed]
- Kelati, A.; Aqil, N.; Baybay, H.; Gallouj, S.; Mernissi, F.Z. Beyond classic dermoscopic patterns of dermatofibromas: A prospective research study. J. Med. Case Rep. 2017, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Zuber, T.J. Punch biopsy of the skin. Am. Fam. Physician 2002, 65, 1155–1158. [Google Scholar] [PubMed]
- Gu, M.; Sohn, K.; Kim, D.; Kim, B. Metastasizing dermatofibroma in lung. Ann. Diagn. Pathol. 2007, 11, 64–67. [Google Scholar] [CrossRef]
- Agero, A.L.C.; Taliercio, S.; Dusza, S.W.; Salaro, C.; Chu, P.; Marghoob, A.A. Conventional and Polarized Dermoscopy Features of Dermatofibroma. Arch. Dermatol. 2006, 142, 1431–1437. [Google Scholar] [CrossRef]
- Zou, M.-H.; Huang, Q.; Yang, T.; Jiang, Y.; Zhang, L.-J.; Xie, Y.; Zheng, R. Role of ultrasound in the diagnosis of primary and recurrent dermatofibrosarcoma protuberans. BMC Cancer 2021, 21, 909. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Słonimska, P.; Sachadyn, P.; Zieliński, J.; Skrzypski, M.; Pikuła, M. Chemotherapy-mediated complications of wound healing: An understudied side effect. Adv. Wound Care 2024, 13, 187–199. [Google Scholar] [CrossRef]
- Kim, E.; Park, H.; Yoon, H.; Cho, S. A case of perforating dermatofibroma with floret-like giant cells. Clin. Exp. Dermatol. 2015, 40, 305–308. [Google Scholar] [CrossRef]
- Ferrari, A.; Argenziano, G.; Buccini, P.; Cota, C.; Sperduti, I.; De Simone, P.; Eibenschutz, L.; Silipo, V.; Zalaudek, I.; Catricalà, C. Typical and atypical dermoscopic presentations of dermatofibroma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1375–1380. [Google Scholar] [CrossRef]
- Bowler, P.; Duerden, B.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Ostapiuk, L. Diagnostic and therapeutic model of sepsis and purulent-Inflammatory diseases. Int. J. Clin. Med. 2019, 10, 577. [Google Scholar] [CrossRef]
- Ng, M.S.; Foong, A.Y.; Koh, M.J. Ulcerated Giant Dermatofibroma following Routine Childhood Vaccination in a Young Boy. Case Rep. Dermatol. 2016, 8, 210–217. [Google Scholar] [CrossRef]
- Aydin, E.; Vardareli, O.S.; Bilezikçi, B.; Ozgirgin, O.N. Dermatofibroma accompanied by perforating dermatosis in the auricle: A case report. Kulak Burun Bogaz Ihtis. Derg. KBB J. Ear Nose Throat 2005, 15, 83–86. [Google Scholar]
- Lungu, A.; Hsieh, A.; Kaya, G.; Menzinger, S. Perforating Fibrous Histiocytoma Mimicking Keratoacanthoma: A Case Report. Dermatopathology 2023, 11, 8–12. [Google Scholar] [CrossRef]
- Abenoza, P.; Lillemoe, T. CD34 and factor XIIIa in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans. Am. J. Dermatopathol. 1993, 15, 429–434. [Google Scholar] [CrossRef]
- Coccolini, F.; Improta, M.; Cicuttin, E.; Catena, F.; Sartelli, M.; Bova, R.; De’Angelis, N.; Gitto, S.; Tartaglia, D.; Cremonini, C.; et al. Surgical site infection prevention and management in immunocompromised patients: A systematic review of the literature. World J. Emerg. Surg. 2021, 16, 33. [Google Scholar] [CrossRef]
- Jung, K.D.; Lee, D.Y.; Lee, J.H.; Yang, J.M.; Lee, E.S. Subcutaneous dermatofibroma. Ann. Dermatol. 2011, 23, 254–257. [Google Scholar] [CrossRef][Green Version]
- Hao, X.; Billings, S.D.; Wu, F.; Stultz, T.W.; Procop, G.W.; Mirkin, G.; Vidimos, A.T. Dermatofibrosarcoma Protuberans: Update on the Diagnosis and Treatment. J. Clin. Med. 2020, 9, 1752. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Trivedi, M.; Reid, D.C. Primary cutaneous blastomycosis after eyebrow wax burn. JAAD Case Rep. 2022, 23, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.V.P.; Matos, D.M.; Barreiros, H.F.; Bártolo, E.A.F.L.F. Variants of dermatofibroma-a histopathological study. An. Bras. Dermatol. 2014, 89, 472–477. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).