Abstract
Introduction: Pasteurella multocida colonizes the oropharynx of various domestic and wild animals. In humans, respiratory tract P. multocida infection is the second most frequent localization and usually manifests as pneumonia. Unilateral absence of pulmonary artery (UAPA) is a very rare congenital anomaly. Adult patients with UAPA are usually asymptomatic or their symptoms are nonspecific. Case report: We report a female patient with hemoptysis admitted to our clinic where we isolated P. multocida in sputum. The organism was also isolated from nasopharyngeal swab of her dog. During hospitalization, she was also diagnosed with UAPA and chronic obstructive pulmonary disease (COPD). Discussion: Respiratory P. multocida infection develops more commonly through contact with animal secretions. It occurs most often in immunocompromised patients and in individuals with comorbidities. Hemoptysis very rarely follows P. multocida infection while it is common in cases of UAPA due to existing developed collateral circulation especially in older patients. Conclusions: Ceased hemoptysis after adequate treatment and no recurrence of it over six years of following up the patient led us to conclude that the cause of hemoptysis was P. multocida infection and not UAPA which was more likely to be the case.
Introduction [1]
Pasteurella multocida is a Gram negative coccobacillus that colonizes the oropharynx of many animal species, most commonly dogs and cats. Respiratory tract P. multocida infection is the second most frequent localization after skin and soft tissue infection. It can manifest as pneumonia, tracheobronchitis, abscess or empyema. It occurs most often in immunocompromised patients and in individuals with comorbidities [1,2,3,4,5].
Congenital unilateral absence of pulmonary artery (UAPA) is a very rare anomaly. It could be isolated or associated with congenital heart defect and seldom gets diagnosed in adults. Adult patients with UAPA are usually asymptomatic or their symptoms are nonspecific [6,7,8].
Hemoptysis can extremely rarely happen in patients with P. multocida infections while it is common in patients with UAPA considering developed collateral circulation in those cases [1,6,8].
We present a female patient with hemoptysis caused by respiratory tract P. multocida infection associated with discovered chronic obstructive pulmonary disease (COPD) and left UAPA.
Case report
A 60-year-old female patient was admitted to our clinic due to hemoptysis followed by weakness and dyspnea. In childhood she had been treated for tuberculosis (TB). She had a 30 pack-year smoking history. She reported no other known illnesses. She was aware of penicillin allergy.
Seven days before admission, she experienced difficulties in terms of dyspnea, intensive fatigue and chest pain. She also reported coughing up 10–20 mL of blood daily starting three days before admission.
Upon admission, the patient was mildly dyspneic and lung auscultation informed of diminished breath sounds on the left side. Her pulse was 84 beats/min and blood pressure was 130/90 mmHg with oxygen saturation of 89% on room air. Laboratory analysis found elevated level of C-reactive protein (CRP) 69 mg/L while the other parameters were within reference ranges. Global respiratory deficiency was seen in arterial blood gases pO2 59 mmHg, pCO2 50 mmHg. Pulmonary function tests revealed very severe obstructive ventilatory defect: forced expiratory volume in one second (FEV1) 24%, forced vital capacity (FVC) 36%, ratio (FEV1/FVC)% 56.4%.
Chest radiography showed slight mediastinum shift to the left, pleuropericardial adhesion on the left, sequelae of TB with no signs of acute infection (Figure 1).
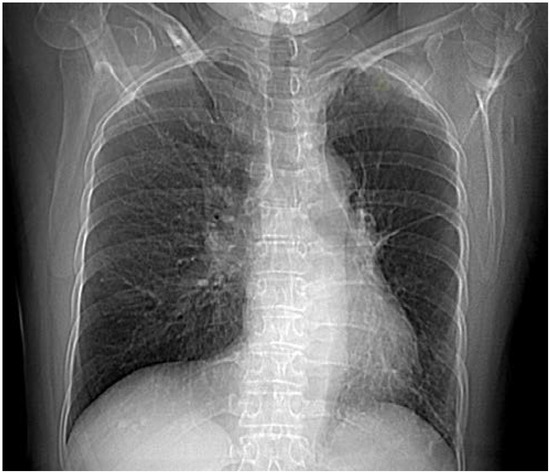
Figure 1.
Chest X-ray showing slight mediastinum shift to the left, pleuropericardial adhesion on the left, diminished left hilum, hyperlucent left lung.
Thorax computed tomography (CT) revealed asymmetry with significantly smaller left lung. The left pulmonary artery immediately after separation was of extremely narrower lumen and so were lobar and segmental branches (Figure 2). Collaterals stemmed from mammary artery and bronchial artery (Figure 3). The results pointed towards UAPA. Subpleurally on the left, apically and basolaterally, scarred changes were noticed.
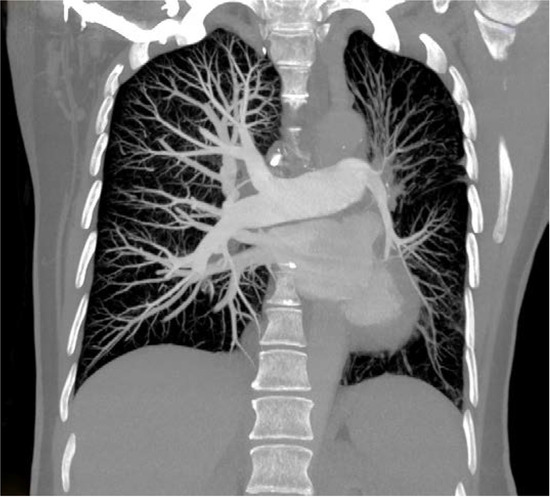
Figure 2.
Computed tomography (CT) showing rudimentary left pulmonary artery.
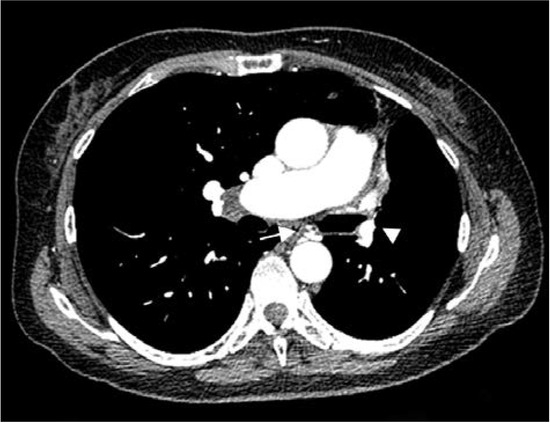
Figure 3.
Computed tomography (CT) showing marked volume loss in left hemithorax, narrow left pulmonary artery (arrowhead), collateral vessel, bronchial artery (arrow).
Bronchoscopy result was with no visible bleeding signs or anomalies.
Smears for acid-fast bacilli were negative.
Heart echocardiogram revealed no associated heart anomalies and right ventricular systolic pressure (RVSP) was 30 mmHg.
Upon admission, treatment was started with empirical antibiotic ceftriaxone intravenously (2 g/day). Sputum analysis, in blood and chocolate agar plates, brought up growths of grayish colonies. They were oxidase and catalase positive. Sputum culture revealed Gram-negative rods and with automated Vitek 2 system using GN cards (BioMérieux, Marcy-l′Étoile, France) they were identified as Pasteurella multocida. Antibiotic sensitivity of bacteria was tested by the disc diffusion method and the E-test method (BioMérieux) was used to determine minimal inhibitory concentrations (MICs). The organism was susceptible to all tested antibiotics, penicillin (MIC = 0.01 μg/mL), amoxicillin-clavulanate (MIC = 0.15 μg/mL), ampicillin (MIC = 0.01 μg/mL), ceftriaxone (MIC = 0.02 μg/mL), levofloxacin (MIC = 0.02 μg/mL), tetracycline (MIC = 0.25 μg/mL), doxycycline (MIC = 0.2 μg/mL), trimethoprim/sulfamethoxazole (MIC = 0.035 μg/mL), azithromycin (MIC = 0.6 μg/mL), and erythromycin (MIC = 0.5 μg/mL).
Treatment was continued according to antibiogram with ceftriaxone for 10 days. On the fifth day upon admission, also according to antibiogram, levofloxacin was added in duration of two weeks, the first ten days intravenously (500 mg/day) and then for the remaining five days orally (500 mg/day). The nasopharyngeal swab tests for members of her family were negative for this bacterium. But P. multocida was isolated from nasopharyngeal swab of her dog. Patient body examination did not expose any scratches or bite marks and she denied any recent scratches or bites that she could recall. Applied antibiotic and bronchodilator therapy resulted in subjective feeling improvement, ceased hemoptysis after three days, CRP normalization after seven days of treatment, gas exchange values improvement (pO2 66 mmHg, pCO2 45 mmHg, oxygen saturation 93%) and lung function improvement (FEV1% 32%, FVC% 64%, (FEV1/FVC)% 43%) on hospital discharge day. The patient was discharged after spending 15 days in our hospital care. Upon hospital release, restricted contact with animals was advised. From there on, she has been undergoing regular follow-up visits and six years after the treatment, the patient shows no signs of hemoptysis.
Discussion
Pasteurella multocida is a facultative anaerobic Gram-negative coccobacillus that colonizes the upper respiratory and digestive tract of various domestic and wild animals [1]. Dogs and cats are the most significant infection transmitters, 70-90% of cats and 20–50% of dogs are colonized by P. multocida [2]. In humans, this bacteria is most commonly associated with soft tissue infection following animal bite or scratch [1]. Infection is manifested as swelling, cellulitis and bloody or suppurative/purulent exudate at the wound site [3].
Respiratory tract is the second most common site of P. multocida infection and this type of infection develops in contact with animal secretions [1,3]. It presents as pneumonia, tracheobronchitis, abscess or empyema with pneumonia being the most frequent [1]. P. multocida can become part of the normal flora of the human respiratory tract. Animal contact at work (in case of veterinarians, butchers, animal breeders, farm and zoo workers) and pet ownership increase the possibility of subclinical carriage or infection [3]. P. multocida infection is more frequent in immunocompromised patients and in individuals with associated pulmonary diseases (COPD, bronchiectasis, pulmonary fibrosis, lung cancer) [1,2,4].
This organism grows in various commercial mediums including sheep blood (the preferred culture medium), chocolate agar, Mueller-Hinton agar but not in MacConkey agar. Most of mediums are catalase, oxidase, indole and ornithine decarboxylase positive. P. multocida identification with biochemistry strips (VITEK2, Minitek) is a quick diagnostic method but can also result in wrong diagnosis. Serotyping based on capsular polysaccharide antigens can help determine the serogroup (A, B, D, E, F). Serotypes A and D are responsible for most infections in humans. Polymerase chain reaction plus sequence based ribotyping superseded previously mentioned P. multocida diagnostic methods [3]. However, next-generation whole genome sequencing is the most accurate method in detecting definitively the source of infection [5].
Penicillin is the medicine of choice in P. multocida treatment and is usually applied in combination with beta-lactamase inhibitor clavulanic acid to overcome possible resistance.
Third generation cephalosporins and fluoroquinolones (levofloxacin) can be implemented in cases of penicillin allergy [4]. The treatment should last at least 10–14 days [1].
Hemoptysis seldom occurs as a consequence of P. multocida infection. Only four cases are reported up to 2018, two of them presenting with lung abscess, one with pneumonia and only one reported hemoptysis as the only P. multocida infection manifestation [1].
Bite mark or a scratch was not noticed in case of our patient; 43% of reported cases of P. multocida infection are not associated with a scratch or a bite [2]. Those infections develop in the nasopharynx or other upper respiratory mucosa through contact with animals or animal secretions or through inhalation with later dissemination [3]. Respiratory infection or bacteremia are more commonly associated with no bite and are more frequent in patients with compelling comorbidities and in older adults [3].
As pet ownership incidence is increasing, possible P. multocida infection needs to be regarded especially in older adults with associated risk factors. Patients need to be asked about animal contacts [1,3].
During hospitalization, our patient was diagnosed with a very severe (GOLD grade 4) COPD, previously untreated, and thorax CT revealed pulmonary artery malformation. Most patients with P. multocida infection have some sort of animal contacts [2], as did our patient who was in daily contact with a colonized dog and considering the dog was only a few months old at the time, our patient shared a bed with her dog occasionally. Hemoptysis occurred three days before the hospital admission.
Unilateral absence of pulmonary artery is a very rare congenital anomaly. It can be isolated or associated with congenital heart defect. The prevalence of isolated anomaly varies from 1 in 200,000 to 1 in 300,000 adults [6,7]. It develops as a consequence of sixth aortic arch malformation during embryogenesis. Proximal interruption of pulmonary artery characterizes UAPA while rudimentary artery points to unilateral hypoplasia of pulmonary artery which is considered a subtype of UAPA [8].
Adult patients with isolated UAPA are usually either asymptomatic or their symptoms are nonspecific. Pulmonary hypertension (PHT) is present in 44% of those cases, hemoptysis in 20%, recurrent pulmonary infections in 37%, limited exercise tolerance in 40% or UAPA can be accidentally discovered upon a chest X-ray [6,7]. Chronic infections can lead to bronchiectasis in some cases. Hemoptysis is associated with apparent collateral circulation [6].
Incidence of hemoptysis increases with age in patients with UAPA. In the study where the median patient age was 14 years the incidence of hemoptysis was 20% while in another study where the median age was 40 years, the incidence of hemoptysis was more than 40% [7,9]. In the latter study of patients with UAPA, hemoptysis was the most common symptom [9]. It is considered that the presence of significant collaterals in older patients increases the possibility of hemoptysis. The most common collaterals stem from bronchial, phrenic, mammary, intercostal arteries, subclavian artery, subdiaphragmatic branches and coronary arteries [6,9]. Hemoptysis can be self-limiting or it can be massive with fatal outcome [7]. In case of our patient, collaterals stemmed from the mammary and bronchial artery.
UAPA diagnosis is complex and requires exams by various medical specialists.
Heart electrocardiogram is normal unless there is PHT when there is a right ventricular dominance [6].
Chest X-ray shows diminished ipsilateral hemithorax with hyperlucent lung. Ipsilateral hemidiaphragm is elevated and the mediastinum is shifted to the affected side with insufficient or missing hilum and hyperinflation of collateral lung [6,7].
Thorax CT reveals abnormal pulmonary artery that can be completely absent or finished 1 cm from its beginning or continuing in very narrow lumen, there can be preserved peripheral branches of the pulmonary artery, variable collateral circulation, possibly associated congenital anomalies, parenchymal changes such as mosaic attenuation and bronchiectasis due to recurrent infections [6,10].
Transthoracic echocardiography excludes other heart and blood vessels anomalies and estimates PHT presence [6].
Treatment of asymptomatic patients or patients with no cardiopulmonary dysfunction is not necessary but they require further monitoring. In case of repeated or massive hemoptysis, treatment can be selective embolization of collateral artery (SECA) [9]. Pneumonectomy is used in cases of massive hemoptysis, SECA failure and frequent and refractory infections. Revascularization in adults is usually not doable considering pulmonary artery changes that are likely narrower or obstructed because of marked fibrosis [9]. In the case of our patient, neither embolization nor surgical treatment was considered because she had no massive hemoptysis that ceased soon after antibiotic treatment was administered.
Conclusions
In the case of our patient, P. multocida infection diagnosis was confirmed presenting with comorbidities such as COPD, TB sequelae as well as pulmonary artery malformation. Performed detailed diagnostics teach us that for adequate diagnosis and optimal treatment a multidisciplinary approach is necessary.
Our patient was treated successfully with antibiotics that resulted in improved clinical parameters and ceased hemoptysis. Considering the absence of PHT and the presence of respiratory infection symptomatology with higher inflammation parameters upon hospital admission in addition to absence of hemoptysis during subsequent regular outpatient follow-up visits, we concluded that the cause of hemoptysis was P. multocida infection.
Author Contributions
RV wrote the article, acquired the microbiological analysis and provided images and interpretation. JJ, ND and JM provided medical treatment, wrote the case history and were involved in the clinical follow-up of the patient. JJ was also involved in writing the article. All authors read and approved the final version of the manuscript.
Funding
None to declare.
Informed Consent Statement
The authors obtained informed consent from the patient for publication of this case report and the accompanying images.
Conflicts of Interest
All authors—none to declare.
References
- Aida, Y.; Kiwamoto, T.; Fujita, K.; et al. Pasteurella multocida pneumonia with hemoptysis: A case report. Respir Med Case Rep 2018, 26, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Dincman, T.; Clyburn, B.E.; Steed, L.L.; Rockey, D.C. Clinical features and outcomes of Pasteurella multocida infection. Medicine (Baltimore) 2015, 94, e1285. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.A.; Ho, M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin Microbiol Rev 2013, 26, 63155. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Treger, K.; Busey, K. Pneumonia and disseminated bacteremia with Pasteurella multocida in the immune competent host: A case report and a review of the literature. Respir Med Case Rep 2015, 15, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Maraki, S.; Kastanis, G.; Stafylaki, D.; Masunt, S.; Kapsetakis, P.; Scoulica, E. Pasteurella multocida wound infection transmitted by a pet dog. Germs 2018, 8, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Kruzliak, P.; Syamasundar, R.P.; Novak, M.; Pechanova, O.; Kovacova, G. Unilateral absence of pulmonary artery: Pathophysiology, symptoms, diagnosis and current treatment. Arch Cardiovasc Dis. 2013, 106, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ten Harkel, A.D.; Blom, N.A.; Ottenkamp, J. Isolated unilateral absence of a pulmonary artery: A case report and review of the literature. Chest 2002, 122, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Emren, S.V.; Tülüce, S.Y.; Tülüce, K. Isolated congenital unilateral agenesis of the left pulmonary artery with left lung hypoplasia in an asymptomatic adult patient. Acta Cardiol Sin 2015, 31, 572–575. [Google Scholar] [PubMed]
- Wang, P.; Yuan, L.; Shi, J.; Xu, Z. Isolated unilateral absence of pulmonary artery in adulthood: A clinical analysis of 65 cases from a case series and systematic review. J Thorac Dis 2017, 9, 4988–4996. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.W.; Lichtenberger, J.P., 3rd; Wu, C.C. Congenital abnormalities of the pulmonary arteries in adults. AJR Am J Roentgenol 2014, 202, W308–W313. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2025.