Molecular Typing, Antibiotic Resistance Profiles and Biocide Susceptibility in Salmonella enterica Serotypes Isolated from Raw Chicken Meat Marketed in Venezuela
Abstract
Introduction
Methods
Salmonella strains
Phenotypic characterization
Genotypic characterization
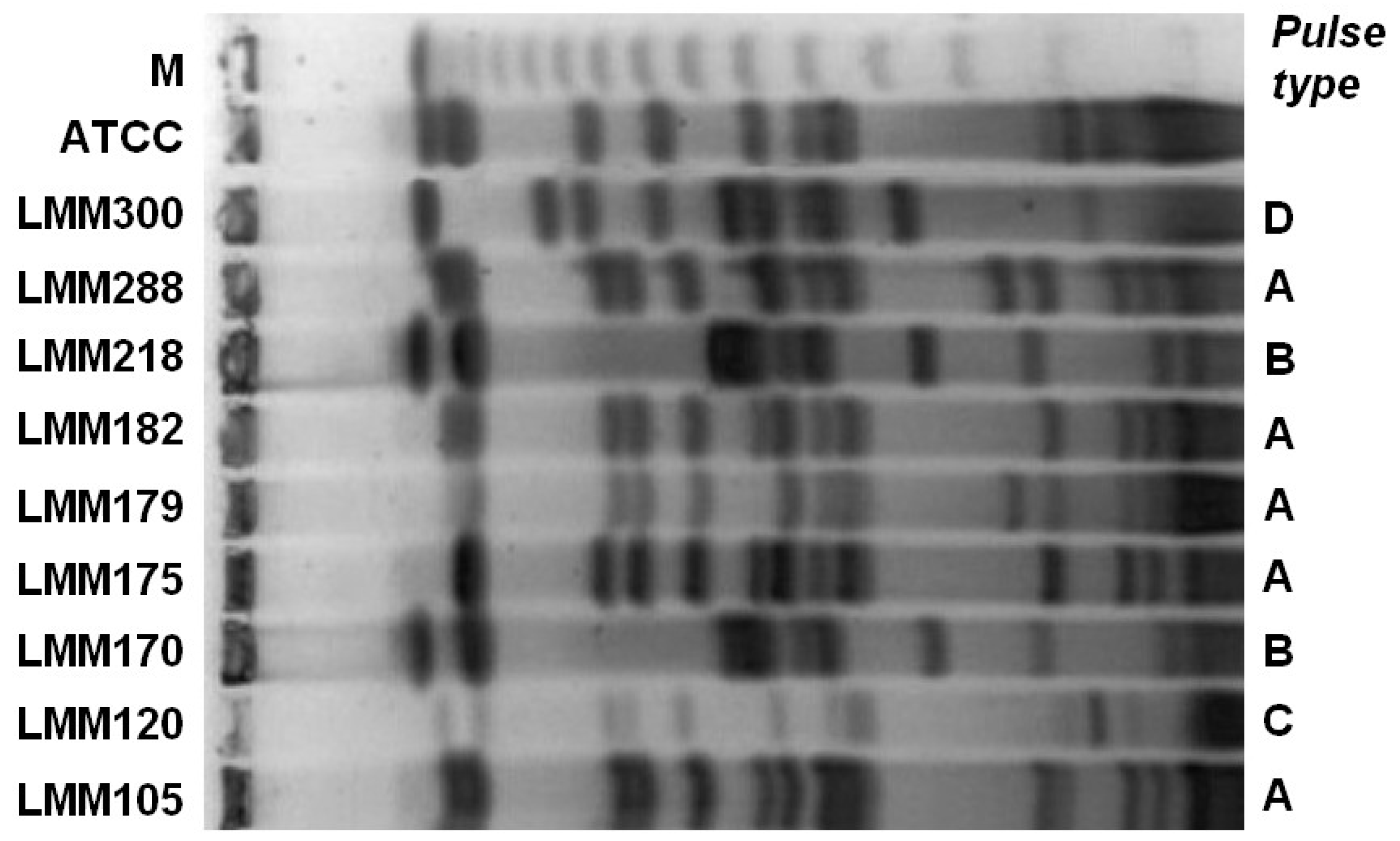
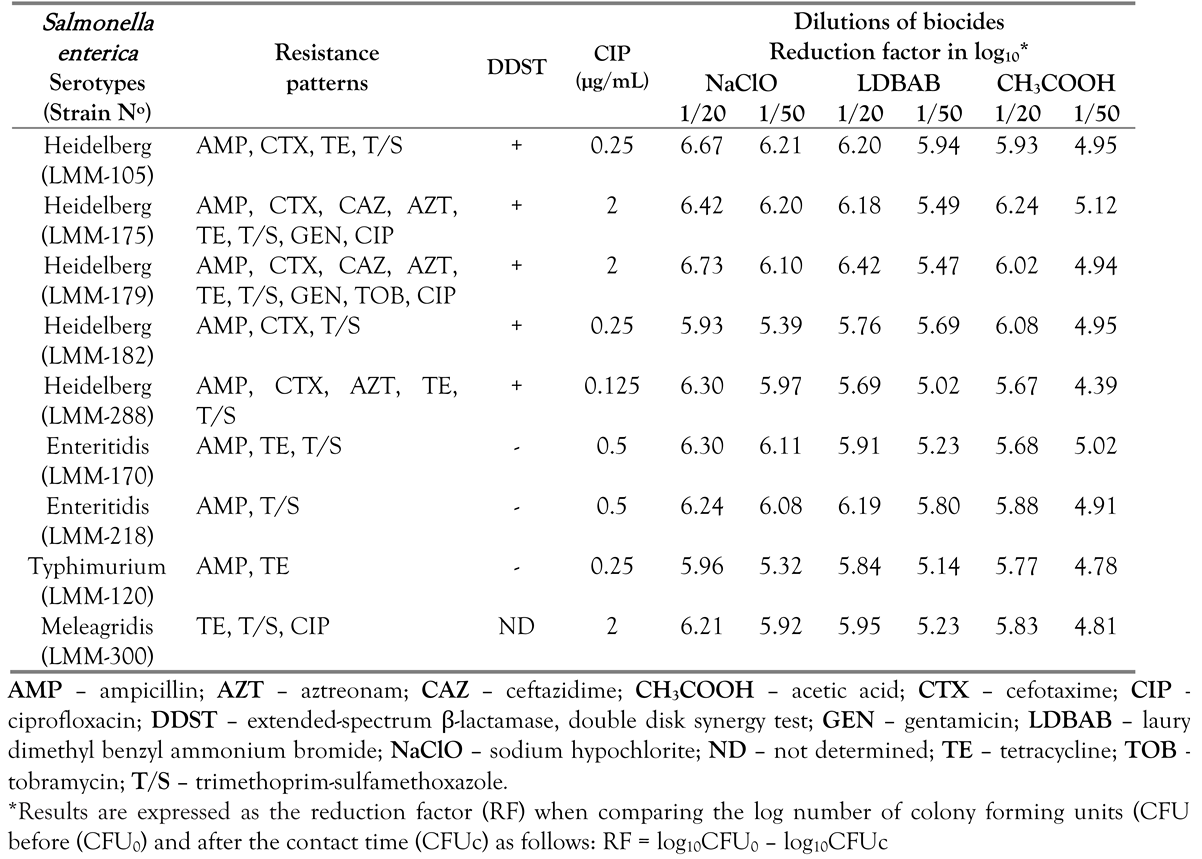
Results
Discussion
Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Su, J.H.; Zhu, Y.H.; Ren, T.Y.; et al. Distribution and antimicrobial resistance of Salmonella isolated from pigs with diarrhea in China. Microorganisms 2018, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elghany, S.; Sallam, K.; Abd-Elkhalek, A.; Tamura, T. Occurrence, genetic characterization and antimicrobial resistance of Salmonella isolated from chicken meat and giblets. Epidemiol Infect 2015, 143, 997–1003. [Google Scholar] [CrossRef]
- Campioni, F.; Moratto Bergamini, A.M.; Falcão, J.P. Genetic diversity, virulence genes and antimicrobial resistance of Salmonella enteritidis isolated from food and humans over a 24-year period in Brazil. Food Microbiol 2012, 32, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Reginatto, G.A.; Ruiz Español, A.; Colantonio, L.D.; Burrone, M.S. [Antimicrobial resistance of Salmonella spp. isolated animal food for human consumption. Rev Peru Med Exp Salud Publica 2016, 33, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Ministerio del Poder Popular para la Salud. Anuario de Mortalidad. Dirección General de Epidemiología. Dirección de Información y Estadística de Salud. 2013. Available online: http://www.mpps.gob.ve/direcciones_msds/epidemiología/estadistica/archivos/anyuarios.htm (accessed on 26 November 2018).
- Rodulfo, H.; De Donato, M.; Luiggi, J.; Michelli, E.; Millán, A.; Michelli, M. Molecular characterization of Salmonella strains in individuals with acute diarrhea syndrome in the State of Sucre, Venezuela. Rev Soc Bras Med Trop 2012, 45, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. ScientificWorldJournal 2015, 2015, 520179. [Google Scholar] [CrossRef]
- Fardsanei, F.; Soltan Dallal, M.M.; Douraghi, M.; et al. Genetic diversity and virulence genes of Salmonella enterica subspecies enterica serotype Enteritidis isolated from meats and eggs. Microb Pathog 2017, 107, 451–456. [Google Scholar] [CrossRef]
- Guiney, D.G.; Fierer, J. The role of the spv genes in Salmonella pathogenesis. Front Microbiol 2011, 2, 129. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Tahoun, A.; El-Gohary, A.E.A. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog 2017, 9, 8. [Google Scholar] [CrossRef]
- González, F.; Araque, M. Association of transferable quinolone resistance determinant qnrB19 with extended-spectrum β-lactamases in Salmonella Give and Salmonella Heidelberg in Venezuela. Int J Microbiol 2013, 2013, 628185. [Google Scholar] [CrossRef]
- Knapp, L.; Amézquita, A.; McClure, P.; Stewart, S.; Maillard, J.Y. Development of a protocol for predicting bacterial resistance to microbicides. Appl Environ Microbiol 2015, 81, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Araque, M. Nontyphoid Salmonella gastroenteritis in pediatric patients from urban areas in the city of Mérida, Venezuela. J Infect Dev Ctries 2009, 3, 28–34. [Google Scholar] [CrossRef] [PubMed]
- González, F.; Pallecchi, L.; Rossolini, G.M.; Araque, M. Plasmid-mediated quinolone resistance determinant qnrB19 in non-typhoidal Salmonella enterica strains isolated in Venezuela. J Infect Dev Ctries 2012, 6, 462–464. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Document M100-S27; LSI: Wayne, PA, USA, 2018. [Google Scholar]
- Asociación Francesa de Normalización; AFNOR. Antiseptiques et désinfectants, 3rd ed.; NF T72-150; AFNOR: París, France, 1995. [Google Scholar]
- Asociación Española de Normalización y Certificación; Antisépticos y desinfectantes químicos; AENOR; Actividad bactericida básica. Método de ensayo y requisites; UNE-EN 1040; AENOR: Madrid, Spain, 1997. [Google Scholar]
- Chiu, C.; Ou, J.T. Rapid identification of Salmonella Serovars in feces by specific detection of virulence genes, invA and spvC, by an enrichment broth culture-multiplex PCR combination assay. J Clin Microbiol 1996, 34, 2619–2622. [Google Scholar] [CrossRef]
- Eaves, D.J.; Randall, L.; Gray, D.T.; et al. Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob Agents Chemother 2004, 48, 4012–4015. [Google Scholar] [CrossRef]
- Flores-Carrero, A.; Labrador, I.; Paniz-Mondolfi, A.; Peaper, D.R.; Towle, D.; Araque, M. Nosocomial outbreak of extended-spectrum β-lactamase-producing Enterobacter ludwigii co-harbouring CTX-M-8, SHV-12 and TEM-15 in a neonatal intensive care unit in Venezuela. J Glob Antimicrob Resist 2016, 7, 114–118. [Google Scholar] [CrossRef]
- Li, L.; Ye, L.; Kromann, S.; Meng, H. Occurrence of extended-spectrum β-lactamases, plasmid-mediated quinolone resistance, and disinfectant resistance genes in Escherichia coli isolated from ready-to-eat meat products. Foodborne Pathog Dis 2017, 142, 109–115. [Google Scholar] [CrossRef]
- Chiou, C.S.; Hong, Y.P.; Liao, Y.S.; et al. New multidrug-resistant Salmonella enterica serovar Anatum clone, Taiwan, 2015-2017. Emerg Infect Dis 2019, 25, 144–147. [Google Scholar] [CrossRef]
- Wales, A.; Davies, R.H. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics 2015, 4, 567–604. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.A.; Hotzel, H.; Awad, O.; et al. Occurrence of Salmonella enterica and Escherichia coli in raw chicken and beef meat in northern Egypt and dissemination of their antibiotic resistance markers. Gut Pathog 2017, 9, 57. [Google Scholar] [CrossRef]
- García, P.; Hopkins, K.L.; García, V.; et al. Diversity of plasmids encoding virulence and resistance functions in Salmonella enterica subsp. enterica serovar Typhimurium monophasic variant 4,[5],12:i:- strains circulating in Europe. PLoS ONE 2014, 9, e89635. [Google Scholar] [CrossRef]
 |
 |
© GERMS 2019.
Share and Cite
González, F.; Araque, M. Molecular Typing, Antibiotic Resistance Profiles and Biocide Susceptibility in Salmonella enterica Serotypes Isolated from Raw Chicken Meat Marketed in Venezuela. GERMS 2019, 9, 81-88. https://doi.org/10.18683/germs.2019.1161
González F, Araque M. Molecular Typing, Antibiotic Resistance Profiles and Biocide Susceptibility in Salmonella enterica Serotypes Isolated from Raw Chicken Meat Marketed in Venezuela. GERMS. 2019; 9(2):81-88. https://doi.org/10.18683/germs.2019.1161
Chicago/Turabian StyleGonzález, Fanny, and María Araque. 2019. "Molecular Typing, Antibiotic Resistance Profiles and Biocide Susceptibility in Salmonella enterica Serotypes Isolated from Raw Chicken Meat Marketed in Venezuela" GERMS 9, no. 2: 81-88. https://doi.org/10.18683/germs.2019.1161
APA StyleGonzález, F., & Araque, M. (2019). Molecular Typing, Antibiotic Resistance Profiles and Biocide Susceptibility in Salmonella enterica Serotypes Isolated from Raw Chicken Meat Marketed in Venezuela. GERMS, 9(2), 81-88. https://doi.org/10.18683/germs.2019.1161




