Vancomycin Resistance Among Staphylococcus aureus Isolates in a Rural Setting, Egypt
Abstract
Introduction
Methods
Collection and processing of samples
Identification and antimicrobial susceptibility
Types of samples and method of selection
Statistical analysis
Results
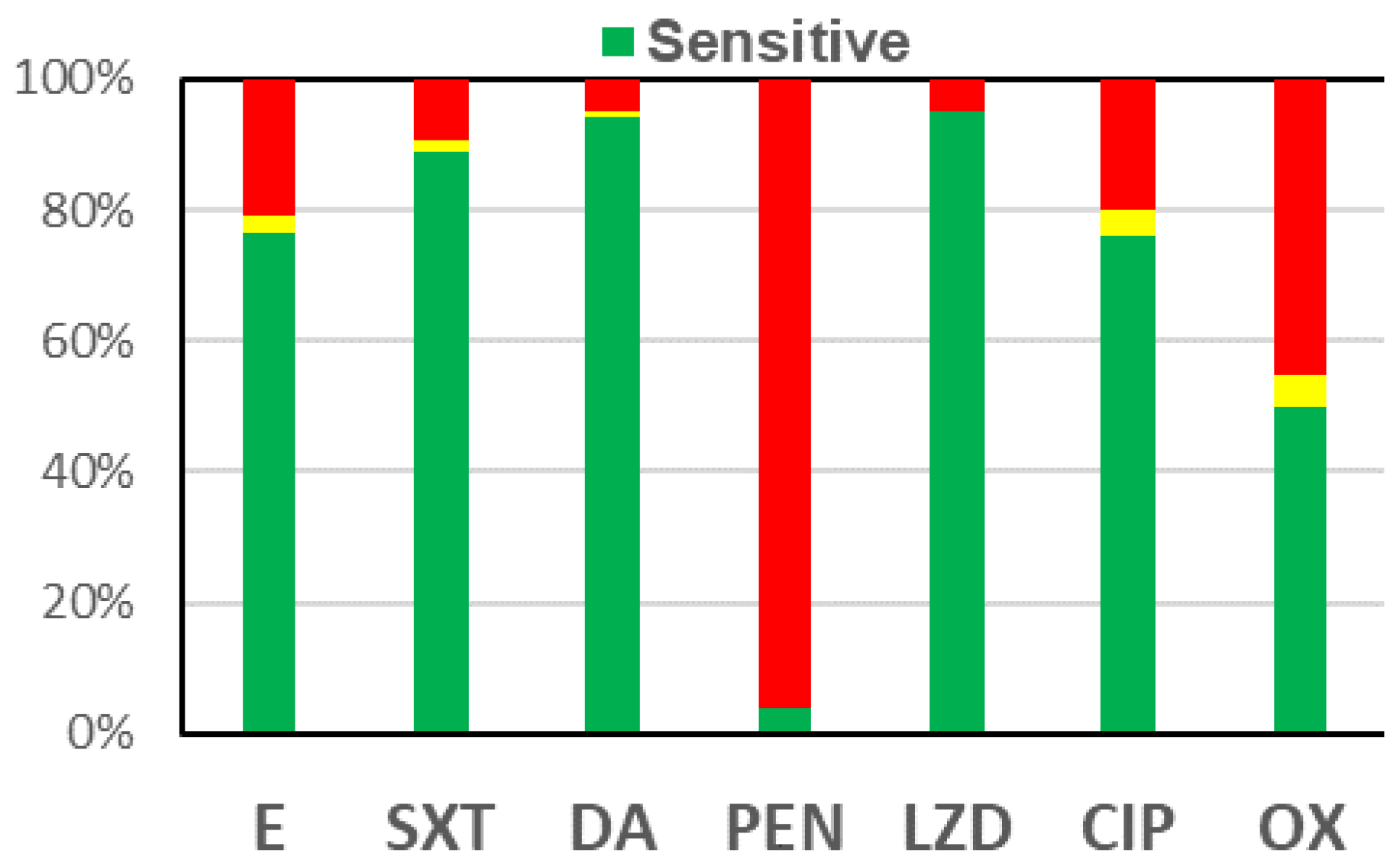
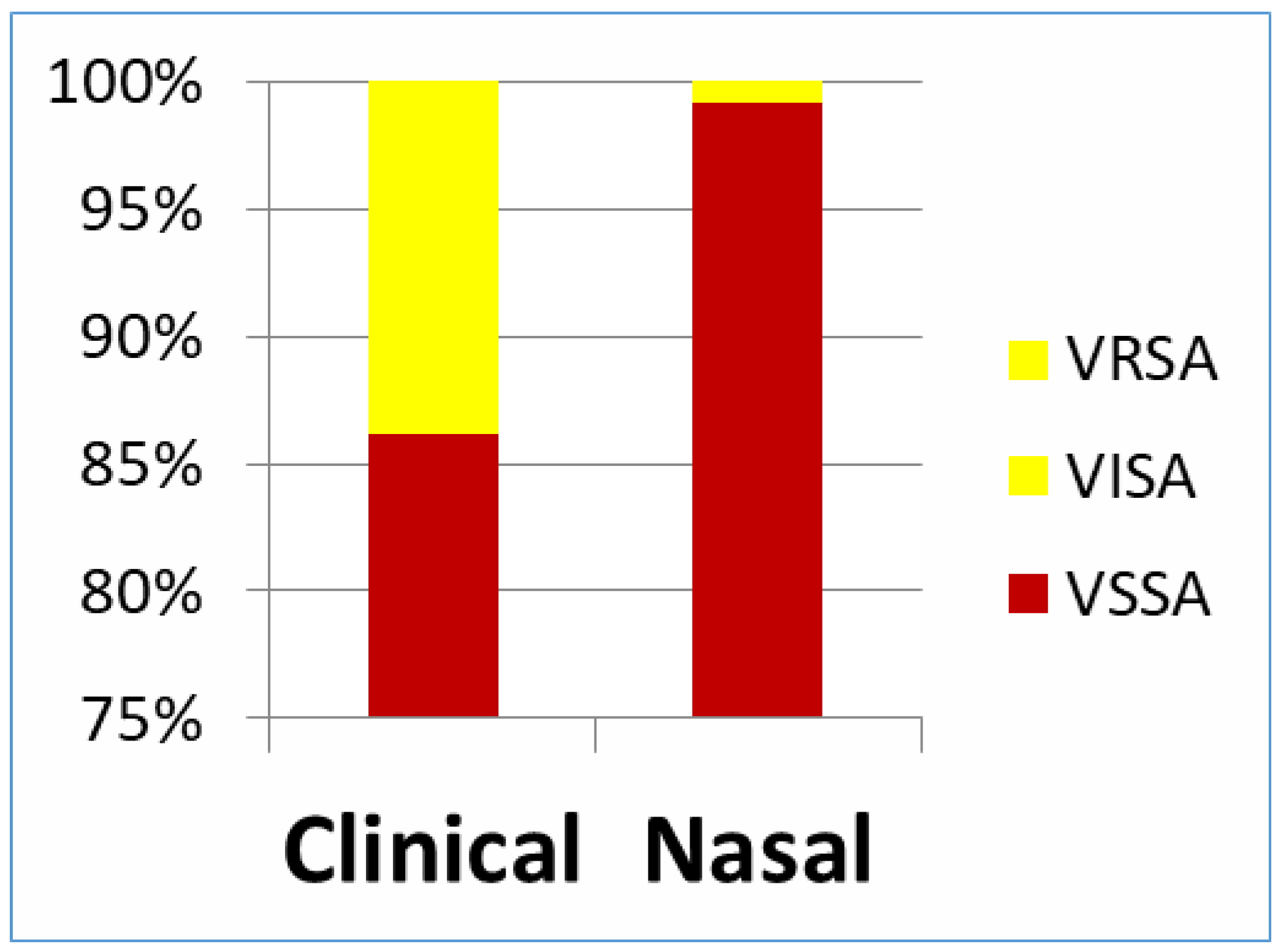
Discussion
Author Contributions
Funding
Conflicts of interest
References
- Dhanalakshmi, T.A.; Umapathy, B.L.; Mohan, D.R. Prevalence of methicillin, vancomycin and multidrug resistance among Staphylococcus aureus. J Clin Diagn Res 2012, 6, 974–977. [Google Scholar]
- Hiramatsu, K.; Aritaka, N.; Hanaki, H.; et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 1997, 350, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Bierbaum, G.; Fuchs, K.; Lenz, W.; Szekat, C.; Sahl, H. Presence of Staphylococcus aureus with reduced susceptibility to vancomycin in Germany. Eur J Clin Microbiol Infect Dis 1999, 18, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.N.; Trivedi, H.R.; Goswami, A.P.; Patel, T.K.; Tripathi, C.B. Antibiotic sensitivity profile of bacterial pathogens in postoperative wound infections at a tertiary care hospital in Gujarat, India. J Pharmacol Pharmacother 2011, 2, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, I.C.V.; Araujo, M.L.C.; Darini, A.L.C. First report of vancomycin-resistant staphylococci isolated from healthy carriers in Brazil. J Clin Microbiol 2005, 43, 179–184. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 15 April 2017).[Green Version]
- NHS Trust. Procedure for Taking a Wound Swab. 2008. Available online: http://www.wirralct.nhs.uk/attachments/article/19/CP23Procedurefortakingawoundswab19Feb13.doc.pdf (accessed on 8 November 2013).[Green Version]
- National Health and Nutrition Examination Survey. Specimen Collection Procedures Manual. 2000. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/specimen_collection_year_3.pdf (accessed on 5 May 2013).[Green Version]
- Forbes, B.; Sahm, D.; Weissfeld, A. Organisms. In Bailey and Scott’s Diagnostic Microbiology, 12th ed.; Forbes, B., Sahm, D., Weissfeld, A., Eds.; Mosby: St. Louis, MO, USA, 2007; pp. 254–264. [Google Scholar][Green Version]
- Clinical and Laboratory Standards Institute. Performance Standards for antimicrobial disk susceptibility Tests; approved standard. 11th ed. M02-A11 ed.; Clinical and Laboratory Standards Institute: Wayne PA, USA, 2012. [Google Scholar][Green Version]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Sobhy, N.; Aly, F.; Abd El Kader, O.; Ghazal, A.; Elbaradei, A. Community-acquired methicillin-resistant Staphylococcus aureus from skin and soft tissue infections (in a sample of Egyptian population): Analysis of mec gene and staphylococcal cassette chromosome. Braz J Infect Dis 2012, 16, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis 2004, 38, S341–S345. [Google Scholar] [CrossRef] [PubMed]
- Moniri, R.; Musav, G.A.; Fadavi, N. The prevalence of nasal carriage methicillin-resistant Staphylococcus aureus in hospitalized patients. Pak J Med Sci 2009, 25, 656–659. [Google Scholar]
- Safdar, N.; Bradley, E. The risk of infection after nasal colonization with Staphylococcus aureus. Am J Med 2008, 121, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fourth informational supplement. M100-S24 ed.; Clinical and Laboratory Standards Institute: Wayne PA, USA, 2014. [Google Scholar]
- Kheder, S.I.; Ali, N.A.; Fathelrahman, A.I. Prevalence and antimicrobial susceptibility pattern of methicillin resistant staphylococci in a Sudanese surgical ward. Pharmacol Pharm 2012, 3, 103–108. [Google Scholar] [CrossRef]
- El-Jakee, J.K.; Atta, N.S.; Samy, A.A.; et al. Antimicrobial resistance in clinical isolates of Staphylococcus aureus from bovine and human sources in Egypt. Glob Vet 2011, 7, 581–586. [Google Scholar]
- Ghoniem, E.M.; El Hendawy, G.R.; Abdel Moteleb, T.M.; Hassan, H.A.; El Refai Khalil, H. Characterization of vancomycin-resistant Staphylococcus aureus in the National Liver Institute. Menoufia Med J 2014, 27, 825–832. [Google Scholar]
- Amr, G.E.; Gammal, S.A. Emergence of vancomycin resistant Staphylococcus aureus isolated from patients in ICUs of Zagazig University Hospitals. Egypt J Med Microbiol 2017, 26, 53–59. [Google Scholar] [CrossRef]
- Amin, M.E.; Amine, A.; Newegy, M.S. Injudicious provision of subtherapeutic doses of antibiotics in community pharmacies. Innov Pharm 2017, 8, 18. [Google Scholar] [CrossRef]
- Okeke, I.N.; Lamikanra, A.; Edelman, R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis 1999, 5, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Ploy, M.C.; Francois, B.; Mounier, M.; Vignon, P.; Denis, F. Nasal carriage of vancomycin-intermediate Staphylococcus aureus among intensive care unit staff. Clin Infect Dis 2001, 33, 1951. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, R.M.A.; Ahmed, H.R.; Gad, G.F.M. Prevalence and conjugal transfer of vancomycin resistance among clinical isolates of Staphylococcus aureus. Adv Res 2014, 2, 12–23. [Google Scholar] [CrossRef]
- El-Daker, M.A.; Mesbah, M.R.; El-Naggar, M.M.; Khalil, E.A.; El-Kenawy, M.F. The first two vancomycin resistant Staphylococcus aureus isolates in Mansoura University Hospital; epidemiology and antimicrobial study. Egypt J Med Microbiol 2008, 17, 31–42. [Google Scholar]
- Sánchez García, M.; De la Torre, M.A.; Morales, G.; et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA 2010, 303, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
| Abscess No (%) | Diabetic foot infection No (%) | Postoperative wound infection No (%) | Skin infection No (%) | Total No (%) | |
|---|---|---|---|---|---|
| S. aureus | 42 (52.5) | 16 (20.0) | 5 (6.2) | 17 (21.3) | 80 (100) |
| Nasal carriage group | S. aureus nasal carriage | Total | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | No. | % | |||
| No. | % | No. | % | |||
| Community | 55 | 12.4 | 387 | 87.6 | 442 | 46.0 |
| Healthcare workers | 28 | 15.6 | 152 | 84.4 | 180 | 18.8 |
| Patients | 37 | 10.9 | 301 | 89.1 | 338 | 35.2 |
| Total | 120 | 12.5 | 840 | 87.5 | 960 | 100.0 |
© GERMS 2018.
Share and Cite
ElSayed, N.; Ashour, M.; Amine, A.E.K. Vancomycin Resistance Among Staphylococcus aureus Isolates in a Rural Setting, Egypt. Germs 2018, 8, 134-139. https://doi.org/10.18683/germs.2018.1140
ElSayed N, Ashour M, Amine AEK. Vancomycin Resistance Among Staphylococcus aureus Isolates in a Rural Setting, Egypt. Germs. 2018; 8(3):134-139. https://doi.org/10.18683/germs.2018.1140
Chicago/Turabian StyleElSayed, Nada, Medhat Ashour, and Amira Ezzat Khamis Amine. 2018. "Vancomycin Resistance Among Staphylococcus aureus Isolates in a Rural Setting, Egypt" Germs 8, no. 3: 134-139. https://doi.org/10.18683/germs.2018.1140
APA StyleElSayed, N., Ashour, M., & Amine, A. E. K. (2018). Vancomycin Resistance Among Staphylococcus aureus Isolates in a Rural Setting, Egypt. Germs, 8(3), 134-139. https://doi.org/10.18683/germs.2018.1140




