Intensive Care Unit-Acquired Acinetobacter baumannii Infections in a Moroccan Teaching Hospital: Epidemiology, Risk Factors and Outcome
Abstract
Introduction
Methods
Study design and setting
Microbiological testing
Statistical analysis
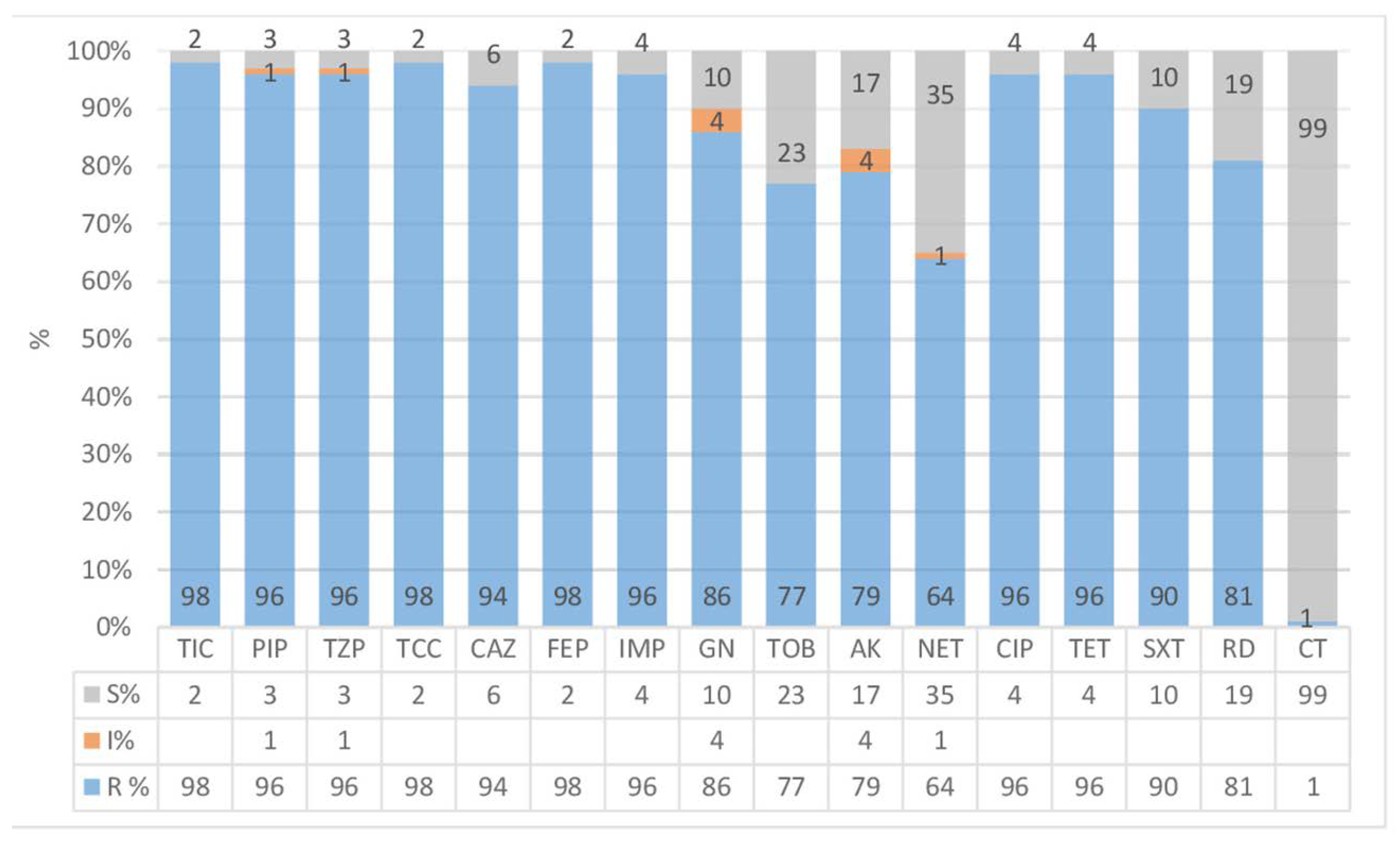
Results
Patient characteristics
Risk factors for ICU acquired A. baumannii infections
Antibiotic treatment of ICU acquired A. baumannii infections
Outcome
Discussion
Conclusion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Parameters | Total N=243 | Cases N=81 | Controls N=162 | P | OR | 95%CI |
|---|---|---|---|---|---|---|
| Male sex, N (%) | 159 (65.4) | 55 (67.9) | 104 (64.2) | 0.567 | 0.8 | 0.5-1.5 |
| Mean age (years), mean ± SD | 58.52±19.5 | 56.75±20.7 | 59.4±18.9 | 0.321 | 1 | 1-1.1 |
| Median length of ICU stay, median [IQR] | 5 [2-14] | 18 [10-26] | 3 [1-6] | <0.0001 | 20.9 | 10.2-42.8 |
| ICU stay ≥14days, N (%) | 61 (25.1) | 51 (63) | 10 (6.2) | <0.0001 | 25.9 | 11.8-56.5 |
| Causes of hospitalization | N (%) | N (%) | N (%) | |||
| Respiratory distress | 48 (19.8) | 16 (19.8) | 32 (19.8) | 1 | 1 | 0.5-2 |
| Consciousness disorder | 24 (9.9) | 9 (11.1) | 15 (9.3) | 0,572 | 1.4 | 0.5-4 |
| Polytrauma | 27 (11.1) | 18 (22.2) | 9 (5.6) | <0.0001 | 4.9 | 2.1-11.4 |
| Post-operative care | 65 (26.7) | 10 (12.3) | 55 (34) | <0.0001 | 0.3 | 0.1-0.6 |
| Acute pancreatitis | 9 (3.7) | 4 (4.9) | 5 (3.1) | 0.471 | 1.6 | 0.4-6.2 |
| Department stay prior to ICU admission | N (%) | N (%) | N (%) | |||
| Emergency | 148 (60.9) | 59 (72.8) | 89 (54.9) | 0.007 | 2.2 | 1.2-3.9 |
| Medical department | 34 (14) | 8 (9.9) | 26 (16) | 0.191 | 0.6 | 0.2-1.3 |
| Surgical department | 61 (25.1) | 18 (22.2) | 43 (26.5) | 0.285 | 0.5 | 0.4-1.5 |
| Underlining disease | N (%) | N (%) | N (%) | |||
| Diabetes | 69 (28.4) | 17 (21) | 52 (32.1) | 0.07 | 0.6 | 0.3-1.1 |
| High blood pressure | 72 (29.6) | 21 (25.9) | 51 (31.5) | 0.371 | 0.8 | 0.4-1.4 |
| Chronic renal failure | 9 (3.7) | 4 (4.9) | 5 (3.1) | 0.471 | 1.6 | 0.4-6.2 |
| Chronic obstructive pulmonary disease | 26 (10.7) | 7 (8.6) | 19 (11.7) | 0.463 | 0.7 | 0.3-1.8 |
| Chronic smoking | 41 (16.9) | 13 (16) | 28 (17.3) | 0.809 | 0.9 | 0.4-1.9 |
| Alcohol abuse | 4 (1.6) | 2 (2.5) | 2 (1.2) | 0.602 | 2.1 | 0.3-14.6 |
| Malignant hemopathies | 8 (3.3) | 4 (4.9) | 4 (2.5) | 0.309 | 2.1 | 0.5-8.4 |
| Previous exposure to invasive procedures | N (%) | N (%) | N (%) | |||
| Arterial catheters | 47 (19.3) | 26 (32.1) | 21 (13) | <0.0001 | 3.2 | 1.6-6.1 |
| Central venous catheters | 59 (24.3) | 42 (51.9) | 17 (10.5) | <0.0001 | 9.2 | 4.7-17.9 |
| Peripheral venous catheters | 54 (22.2) | 21 (25.9) | 33 (20.4) | 0.326 | 1.4 | 0.7-2.6 |
| Urinary catheter | 78 (32.1) | 50 (61.7) | 28 (17.3) | <0.0001 | 7.7 | 4.2-14.1 |
| Nasogastric tube | 14 (5.8) | 9 (11.1) | 5 (3.1) | 0.018 | 3.9 | 1.3-12.1 |
| Mechanical ventilation | 105 (43.2) | 60 (74.1) | 45 (27.8) | <0.0001 | 7.4 | 4.1-13.6 |
| Chest tube | 11 (4.5) | 4 (4.9) | 7 (4.3) | 0.827 | 1.1 | 0.3-4 |
| Recent surgery | 45 (18.5) | 12 (14.8) | 33 (20.4) | 0.293 | 0.7 | 0.3-1.4 |
| Parenteral nutrition | 62 (25.5) | 45 (55.6) | 17 (10.5) | <0.0001 | 1.2 | 5.5-20.8 |
| Dialysis | 8 (3.3) | 3 (3.7) | 5 (3.1) | 0.799 | 1.2 | 0.3-5.2 |
| Hemodialysis | 8 (3.3) | 4 (4.9) | 4 (2.5) | 0.309 | 2.1 | 0.5-8.4 |
| Invasive procedures ≥7 days | 81 (52.6) | 61 (81.4) | 20 (25) | <0.0001 | 14.1 | 6.4-30.8 |
| Clinical complications | N (%) | N (%) | N (%) | |||
| Sepsis | 6 (2.5) | 3 (3.7) | 3 (1.9) | 0.381 | 2 | 0.4-10.3 |
| Severe sepsis | 11 (4.5) | 6 (7.4) | 5 (3.1) | 0.187 | 2.5 | 0.7-8.5 |
| Septic shock | 93 (38.3) | 55 (67.9) | 38 (23.5) | <0.0001 | 6.9 | 3.8-12.5 |
| Empirical antibiotic therapy | 192 (79) | 79 (95.7) | 113 (69.8) | <0.0001 | 17.1 | 4-72.5 |
| Penicillins | 52 (21.4) | 18 (22.2) | 34 (21) | 0.825 | 1.1 | 0.6-2.1 |
| Third generation cephalosporins | 89 (36.6) | 38 (46.9) | 51 (31.5) | 0.019 | 1.9 | 1.1-3.3 |
| Imipenem | 94 (38.7) | 62 (76.5) | 32 (19.8) | <0.0001 | 13.3 | 7-25.2 |
| Amikacin | 84 (34.6) | 49 (60.5) | 35 (21.6) | <0.0001 | 5.6 | 3.1-9.9 |
| Gentamicin | 15 (6.2) | 7 (8.6) | 8 (4.9) | 0.258 | 1.8 | 0.6-5.2 |
| Quinolones | 33 (13.6) | 9 (11.1) | 24 (14.8) | 0.427 | 0,7 | 0.3-1.6 |
| Glycopeptide antibiotics | 18 (7.4) | 14 (17.2) | 4 (2.5) | <0.0001 | 7.6 | 2.4-24 |
| Metronidazole | 32 (13.2) | 12 (14.8) | 20 (12.3) | 0.592 | 1.2 | 0.6-2.7 |
| Combination antibiotic therapy | N (%) | N (%) | N (%) | |||
| Mono-antimicrobial therapy | 40 (16.5) | 9 (11.1) | 31 (19.1) | 0.112 | 0.5 | 0.2-1.2 |
| Bi-antimicrobial therapy | 53 (21.8) | 17 (21) | 36 (22.2) | 0.826 | 0.9 | 0.5-1.8 |
| Antibiotic polytherapy | 94 (38.7) | 50 (61.7) | 44 (27.2) | <0.0001 | 4.3 | 2.4-7.6 |
| Corticotherapy | 107 (44) | 50 (61.7) | 57 (35.2) | <0.0001 | 3 | 1.7-5.2 |
| Empirical antibiotic treatment ≥5 days | 105 (54.4) | 66 (82.5) | 39 (34.5) | <0.0001 | 8.9 | 4.5-17.9 |
| Mortality rate | 105 (43.2) | 60 (74.1) | 45 (27.3) | <0.0001 | 7.4 | 4.1-13.6 |
| Parameters | p | OR | 95%CI |
| Duration of ICU stay ≥14 days | 0.048 | 6.4 | 1.1-41 |
| Admission for polytrauma | 0.266 | 3.9 | 0.3-44.5 |
| Previous hospitalization in Emergency department | 0.353 | 0.5 | 0.1-2.3 |
| Prior exposure to arterial catheters | 0.060 | 0.2 | 0.1-1.1 |
| Previous use of central venous catheters | 0.006 | 18 | 2.3-141.5 |
| Previous exposure to urinary catheter | 0.152 | 0.3 | 0.1-1.5 |
| Prior use of nasogastric tube | 0.150 | 6.9 | 0.5-95.5 |
| Previous exposure to mechanical ventilation | 0.003 | 9.5 | 2.1-42.6 |
| Previous exposure to parenteral nutrition | 0.411 | 1.9 | 0.4-9.1 |
| Duration of invasive procedures ≥7 days | 0.033 | 7.8 | 1.2-51.2 |
| History of septic shock | 0.919 | 0.9 | 0.2-3.8 |
| Prior use of antibiotic therapy | 0.286 | 2.4 | 0.5-12.4 |
| Previous use of third generation cephalosporins | 0.066 | 4.2 | 0.9-19.5 |
| Prior exposure to imipenem | 0.012 | 9.1 | 1.6-51.5 |
| Prior use of amikacin | 0.027 | 5.2 | 1.2-22.4 |
| Prior use of glycopeptide antibiotics | 0.279 | 3.6 | 0.4-37.4 |
| Prior exposure to antibiotic polytherapy | 0.003 | 11.8 | 2.3-60.3 |
| Duration of empirical antibiotic treatment ≥5 days | 0.324 | 0.4 | 0.1-2.8 |
| Previous corticotherapy | 0.029 | 5 | 1.2-21.3 |
| Variables | Deceased | Survivors | p |
|---|---|---|---|
| N=60 | N=21 | ||
| Male sex, N (%) | 39 (65) | 16 (76.2) | 0.344 |
| Mean age (years), mean±SD | 60.4±19.2 | 46.63±21.7 | 0.362 |
| Age ≥65 years, N (%) | 27 (45) | 4 (19) | 0.035 |
| Median duration of hospitalization before diagnosis of infection, median [IQR] | 7.5 [4.25-12] | 7 [4.5-12] | 0.838 |
| ICU stay ≥14days, N (%) | 35 (58.3) | 16(76.2) | 0.145 |
| Causes of hospitalization | N (%) | N (%) | |
| Respiratory distress | 13 (21.7) | 3 (14.3) | 0.543 |
| Consciousness disorder | 10 (16.7) | 3 (14.3) | 1 |
| Polytrauma | 13 (21.7) | 5 (23.8) | 0.839 |
| Post-operative care | 7 (11.7) | 3 (14.3) | 0.714 |
| Department stay prior to ICU admission | N (%) | N (%) | |
| Emergency | 42 (70) | 17 (81) | 0.331 |
| Medical department | 7 (11.7) | 1 (4.8) | 0.673 |
| Surgical department | 11 (11.8) | 3 (14.3) | 1 |
| Underlining disease | N (%) | N (%) | |
| Diabetes | 13 (21.7) | 4 (19) | 0.8 |
| High blood pressure | 17 (28.3) | 4 (19) | 0.403 |
| Chronic renal failure | 3 (5) | 1 (4.8) | 1 |
| Chronic obstructive pulmonary disease | 4 (6.7) | 3 (14.3) | 0.368 |
| Chronic smoking | 10 (16.7) | 3 (14.3) | 1 |
| Alcohol abuse | 2 (3.3) | 0 | 1 |
| Malignant hemopathies | 4 (6.7) | 0 | 0.568 |
| Invasive procedures | N (%) | N (%) | |
| Arterial catheters | 19 (31.7) | 7 (33.3) | 1 |
| Central venous catheters | 32 (53.3) | 10 (47.6) | 0.652 |
| Peripheral venous catheters | 16 (26.7) | 5 (23.8) | 0.797 |
| Urinary catheter | 38 (63.3) | 12 (57.1) | 0.615 |
| Nasogastric tube | 5 (8.3) | 4 (19) | 0.228 |
| Mechanical ventilation | 46 (76.7) | 14 (66.7) | 0.368 |
| Recent surgery | 7 (11.7) | 5 (23.8) | 0.282 |
| Parenteral nutrition | 34 (56.7) | 11 (52.4) | 0.734 |
| Invasive procedures ≥7 days | 45 (81.8) | 16 (84.2) | 1 |
| Clinical complications | N (%) | N (%) | |
| Sepsis | 3 (5) | 0 | 0.564 |
| Severe sepsis | 4 (6.7) | 2 (9.5) | 0.647 |
| Septic shock | 50 (83.3) | 5 (23.8) | <0.0001 |
| Empirical antibiotic therapy | 59 (98.3) | 20 (95.2) | 0.454 |
| Beta lactam antibiotics | 59 (98.3) | 21 (100) | 1 |
| Aminoglycosides | 41 (68.3) | 12 (57.1) | 0.353 |
| Quinolones | 7 (11.7) | 2 (9.5) | 0.788 |
| Combination antibiotic therapy | N (%) | N (%) | |
| Mono-antimicrobial therapy | 7 (11.7) | 2 (9.5) | 0.788 |
| Bi-antimicrobial therapy | 16 (26.7) | 1 (4.8) | 0.034 |
| Antibiotic polytherapy | 35 (58.3) | 15 (71.4) | 0.288 |
| Corticotherapy | 40 (66.7) | 10 (47.6) | 0.122 |
| Empirical antibiotic treatment ≥5 days | 49 (83.1) | 17 (81) | 1 |
| Presence of co-infections with A. baumannii | 33 (55) | 13 (61.9) | 0.582 |
| Category of resistance | N (%) | N (%) | |
| MDR | 57 (95) | 20 (95.2) | 0.965 |
| XDR | 27 (45) | 11 (52.4) | 0.56 |
| Targeted antibiotic therapy, N (%) | 49 (81.7) | 16 (76.2) | 0.4 |
| Colistin, N (%) | 40 (66.7) | 15 (71.4) | 0.687 |
| Amikacin, N (%) | 15 (25) | 3 (14.3) | 0.376 |
| Duration of targeted antibiotic therapy, | 10.58±7.9 | 15.53±11.4 | 0.059 |
| Parameters | P | OR | 95%CI |
| Septic shock | <0.0001 | 19.2 | 5.2-71.4 |
| Age ≥65 years | 0.031 | 4.9 | 1.1-21.1 |
References
- Kalanuria, A.A.; Ziai, W.; Mirski, M. Ventilator-associated pneumonia in the ICU. Crit Care 2014, 18, 208. [Google Scholar]
- Timsit, J.F.; Soubirou, J.F.; Voiriot, G.; et al. Treatment of bloodstream infections in ICUs. BMC Infect Dis 2014, 14, 489. [Google Scholar]
- Uwingabiye, J.; Frikh, M.; Lemnouer, A.; et al. Acinetobacter infections prevalence and frequency of the antibiotics resistance: comparative study of intensive care units versus other hospital units. Pan Afr Med J 2016, 23, 191. [Google Scholar]
- Uwingabiye, J.; Lemnouer, A.; Roca, I.; et al. Clonal diversity and detection of carbapenem resistance encoding genes among multidrug-resistant Acinetobacter baumannii isolates recovered from patients and environment in two intensive care units in a Moroccan hospital. Antimicrob Resist Infect Control 2017, 6, 99. [Google Scholar] [PubMed]
- Lanjri, S.; Uwingabiye, J.; Frikh, M.; et al. In vitro evaluation of the susceptibility of Acinetobacter baumannii isolates to antiseptics and disinfectants: comparison between clinical and environmental isolates. Antimicrob Resist Infect Control 2017, 6, 36. [Google Scholar] [PubMed]
- Lin, M.F.; Lan, C.Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J Clin Cases 2014, 2, 787–814. [Google Scholar]
- Elouennass, M.; Bajou, T.; Lemnouer, A.H.; Foissaud, V.; Hervé, V.; Baaj, A.J. Acinetobacter baumannii: étude de la sensibilité des souches isolées à l'hôpital militaire d'instruction Mohammed V, Rabat, Maroc. Med Mal Infect 2003, 33, 361–364. [Google Scholar]
- Punpanich, W.; Nithitamsakun, N.; Treeratweeraphong, V.; Suntarattiwong, P. Risk factors for carbapenem non- susceptibility and mortality in Acinetobacter baumannii bacteremia in children. Int J Infect Dis 2012, 16, e811–e815. [Google Scholar]
- Özgür, E.S.; Horasan, E.S.; Karaca, K.; Ersöz, G.; Naycı Atış, S.; Kaya, A. Ventilator-associated pneumonia due to extensive drug-resistant Acinetobacter baumannii: risk factors, clinical features, and outcomes. Am J Infect Control 2014, 42, 206–208. [Google Scholar] [PubMed]
- Townsend, J.; Park, A.N.; Gander, R.; et al. Acinetobacter infections and outcomes at an academic medical center: a disease of long-term care. Open Forum Infect Dis 2015, 2, ofv023. [Google Scholar]
- Liu, Q.; Li, W.; Du, X.; et al. Risk and prognostic factors for multidrug-resistant Acinetobacter baumannii complex bacteremia: a retrospective study in a tertiary hospital of west China. PLoS One 2015, 10, e0130701. [Google Scholar]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008, 36, 309–332. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; et al. Multidrug- resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012, 18, 268–281. [Google Scholar]
- Mathai, A.S.; Oberoi, A.; Madhavan, S.; Kaur, P. Acinetobacter infections in a tertiary level intensive care unit in northern India: epidemiology, clinical profiles and outcomes. J Infect Public Health 2012, 5, 145–152. [Google Scholar] [PubMed]
- Ñamendys-Silva, S.A.; Correa-García, P.; García-Guillén, F.J.; et al. Outcomes of critically ill cancer patients with Acinetobacter baumannii infection. World J Crit Care Med 2015, 4, 258–264. [Google Scholar]
- Cisneros, J.M.; Rodríguez-Baño, J.; Fernández-Cuenca, F.; et al. Risk-factors for the acquisition of imipenem-resistant Acinetobacter baumannii in Spain: a nationwide study. Clin Microbiol Infect 2005, 11, 874–879. [Google Scholar] [PubMed]
- Chopra, T.; Marchaim, D.; Johnson, P.C.; et al. Risk factors and outcomes for patients with bloodstream infection due to Acinetobacter baumannii-calcoaceticus complex. Antimicrob Agents Chemother 2014, 58, 4630–4635. [Google Scholar] [PubMed]
- Ardoino, I.; Zangirolami, F.; Iemmi, D.; et al. Risk factors and epidemiology of Acinetobacter baumannii infections in a university hospital in Northern Italy: A case-control study. Am J Infect Control 2016, 44, 1600–1605. [Google Scholar]
- Turkoglu, M.; Mirza, E.; Tunçcan, O.G.; et al. Acinetobacter baumannii infection in patients with hematologic malignancies in intensive care unit: risk factors and impact on mortality. J Crit Care 2011, 26, 460–467. [Google Scholar]
- García-Garmendia, J.L.; Ortiz-Leyba, C.; Garnacho-Montero, J.; et al. Risk factors for Acinetobacter baumannii nosocomial bacteremia in critically Ill patients: a cohort study. Clin Infect Dis 2001, 33, 939–946. [Google Scholar]
- Manchanda, V.; Sanchaita, S.; Singh, N. Multidrug resistant Acinetobacter. J Glob Infect Dis 2010, 291–304. [Google Scholar]
- Arefian, H.; Hagel, S.; Heublein, S.; et al. Extra length of stay and costs because of health care-associated infections at a German university hospital. Am J Infect Control 2016, 44, 160–166. [Google Scholar]
- Sunenshine, R.H.; Wright, M.O.; Maragakis, L.L.; et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 2007, 13, 97–103. [Google Scholar]
- Jung, J.Y.; Park, M.S.; Kim, S.E.; et al. Risk factors for multi- drug resistant Acinetobacter baumannii bacteremia in patients with colonization in the intensive care unit. BMC Infect Dis 2010, 10, 228. [Google Scholar]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 2012, 25, 450–470. [Google Scholar]
- Ng, T.M.; Teng, C.B.; Lye, D.C.; Apisarnthanarak, A. A multicenter case-case control study for risk factors and outcomes of extensively drug-resistant Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol 2014, 35, 49–55. [Google Scholar]
- Lee, S.O.; Kim, N.J.; Choi, S.H.; et al. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: a case-control study. Antimicrob Agents Chemother 2004, 48, 224–228. [Google Scholar] [PubMed]
- Lachhab, Z.; Frikh, M.; Maleb, A.; et al. Bacteraemia in intensive care unit: clinical, bacteriological, and prognostic prospective study. Can J Infect Dis Med Microbiol 2017, 2017, 4082938. [Google Scholar]
- Kassamali, Z.; Jain, R.; Danziger, L.H. An update on the arsenal for multidrug-resistant Acinetobacter infections: polymyxin antibiotics. Int J Infect Dis 2015, 30, 125–132. [Google Scholar] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, M.; et al. Biology of Acinetobacter baumannii: pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front Cell Infect Microbiol 2017, 7, 55. [Google Scholar]
- del Mar Tomas, M.; Cartelle, M.; Pertega, S.; et al. Hospital outbreak caused by a carbapenem-resistant strain of Acinetobacter baumannii: patient prognosis and risk- factors for colonisation and infection. Clin Microbiol Infect 2005, 11, 540–546. [Google Scholar] [PubMed]
- Nasa, P.; Juneja, D.; Singh, O. Severe sepsis and septic shock in the elderly: An overview. World J Crit Care Med 2012, 1, 23–30. [Google Scholar] [PubMed]
| Parameters | N | % |
|---|---|---|
| Gram-negative bacilli | 42 | 71.1 |
| Non-fermenting Gram-negative bacilli | 22 | 37.2 |
| Pseudomonas spp. | 21 | 35.6 |
| Stenotrophomonas maltophilia | 1 | 1.7 |
| Enterobacteriaceae | 18 | 30.5 |
| Escherichia coli | 4 | 6.7 |
| Klebsiella pneumoniae | 7 | 11.9 |
| Enterobacter spp. | 4 | 6.7 |
| Serratia spp. | 1 | 1.7 |
| Proteus spp. | 2 | 3.4 |
| Other Gram-negative bacilli | 2 | 3.4 |
| Haemophilus influenzae | 2 | 3.4 |
| Gram-positive cocci | 13 | 22 |
| Staphylococcus aureus | 8 | 13.5 |
| Coagulase-negative staphylococci | 2 | 3.4 |
| Enterococcus faecalis | 1 | 1.7 |
| Streptococcus spp. | 2 | 3.4 |
| Gram-positive bacilli | 4 | 6.7 |
| Corynebacterium spp. | 4 | 6.7 |
| Total | 59 | 100 |
© GERMS 2017.
Share and Cite
Uwingabiye, J.; Lemnouer, A.; Baidoo, S.; Frikh, M.; Kasouati, J.; Maleb, A.; Benlahlou, Y.; Bssaibis, F.; Mbayo, A.; Doghmi, N.; et al. Intensive Care Unit-Acquired Acinetobacter baumannii Infections in a Moroccan Teaching Hospital: Epidemiology, Risk Factors and Outcome. Germs 2017, 7, 193-205. https://doi.org/10.18683/germs.2017.1126
Uwingabiye J, Lemnouer A, Baidoo S, Frikh M, Kasouati J, Maleb A, Benlahlou Y, Bssaibis F, Mbayo A, Doghmi N, et al. Intensive Care Unit-Acquired Acinetobacter baumannii Infections in a Moroccan Teaching Hospital: Epidemiology, Risk Factors and Outcome. Germs. 2017; 7(4):193-205. https://doi.org/10.18683/germs.2017.1126
Chicago/Turabian StyleUwingabiye, Jean, Abdelhay Lemnouer, Sabina Baidoo, Mohammed Frikh, Jalal Kasouati, Adil Maleb, Yassine Benlahlou, Fatna Bssaibis, Albert Mbayo, Nawfal Doghmi, and et al. 2017. "Intensive Care Unit-Acquired Acinetobacter baumannii Infections in a Moroccan Teaching Hospital: Epidemiology, Risk Factors and Outcome" Germs 7, no. 4: 193-205. https://doi.org/10.18683/germs.2017.1126
APA StyleUwingabiye, J., Lemnouer, A., Baidoo, S., Frikh, M., Kasouati, J., Maleb, A., Benlahlou, Y., Bssaibis, F., Mbayo, A., Doghmi, N., Abouelalaa, K., Baite, A., Ibrahimi, A., & Elouennass, M. (2017). Intensive Care Unit-Acquired Acinetobacter baumannii Infections in a Moroccan Teaching Hospital: Epidemiology, Risk Factors and Outcome. Germs, 7(4), 193-205. https://doi.org/10.18683/germs.2017.1126




