Endocrine Dysfunction in Sepsis: A Beneficial or Deleterious Host Response?
Abstract
Introduction
Search criteria
Literature review
Interrelation Between the Central Nervous System and the Immune System During Sepsis
Hypothalamic-Pituitary-Adrenal Axis in Sepsis
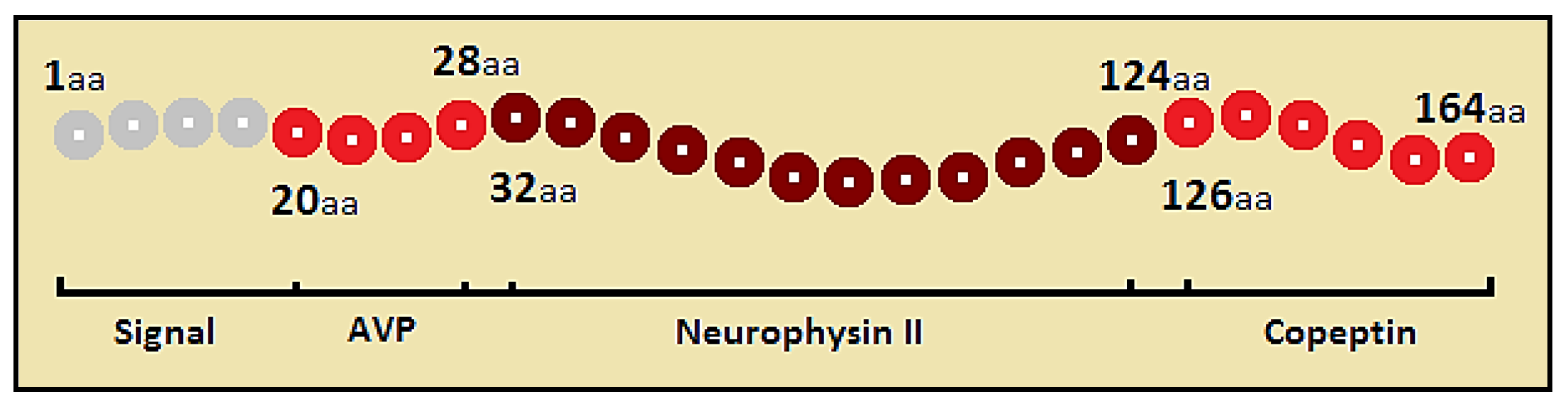
Vasopressin
Copeptin
Cortisol
Glucose Metabolism in Sepsis
Leptin
Hypothalamo-Pituitary-Thyroid Axis in Sepsis
Conclusions
Acknowledgments
Conflicts of Interest
References
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Chen, M.; Wang, B.; Xu, Y.; et al. Diagnostic value of serum leptin and a promising novel diagnostic model for sepsis. Exp. Ther. Med. 2014, 7, 881–886. [Google Scholar] [CrossRef]
- Munford, R.S.; Tracey, K.J. Is severe sepsis a neuroendocrine disease? Mol. Med. 2002, 8, 437–442. [Google Scholar] [CrossRef]
- Sharshar, T.; Hopkinson, N.S.; Orlikowski, D.; Annane, D. Science review: The brain in sepsis—Culprit and victim. Crit. Care 2005, 9, 37–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choong, K. Hormonal therapies in severe sepsis. In Severe Sepsis and Septic Shock—Understanding a Serious Killer; Fernandez, R., Ed.; InTech: London, UK, 2012; pp. 359–378. [Google Scholar][Green Version]
- Khardori, R.; Castillo, D. Endocrine and metabolic changes during sepsis: An update. Med. Clin. North. Am. 2012, 96, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Christ-Crain, M. The stress hormone copeptin: A new prognostic biomarker in acute illness. Swiss Med. Wkly. 2010, 140, w13101. [Google Scholar] [PubMed]
- Van der Berghe, G.; de Zegher, F.; Bouillon, R. Clinical review 95: Acute and prolonged critical illness as different neuroendocrine paradigms. J. Clin. Endocrinol. Metab. 1998, 83, 1827–1834. [Google Scholar]
- Cooper, M.S.; Stewart, P.M. Corticosteroid insufficiency in acutely ill patients. N. Engl. J. Med. 2003, 348, 727–734. [Google Scholar] [CrossRef]
- Marik, P.E.; Pastores, S.M.; Annane, D.; et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine. Crit. Care Med. 2008, 36, 1937–1949. [Google Scholar] [CrossRef]
- Menon, K.; Ward, R.E.; Lawson, M.L.; et al. A prospective multicenter study of adrenal function in critically ill children. Am. J. Respir. Crit. Care Med. 2010, 182, 246–251. [Google Scholar] [CrossRef]
- Tordman, K.; Jaffe, A.; Trostanetsky, Y.; Greenman, Y.; Limor, R.; Stern, N. Low-dose (1 microgram) adrenocorticotrophin (ACTH) stimulation as a screening test for impaired hypothalamo-pituitary-adrenal axis function: Sensitivity, specificity and accuracy in comparison with the high-dose (250 microgram) test. Clin. Endocrinol. (Oxf.) 2000, 52, 633–640. [Google Scholar] [CrossRef]
- Lipiner-Frieman, D.; Sprung, C.L.; Laterre, P.F.; et al. Adrenal function in sepsis: The retrospective Corticus study. Crit. Care Med. 2007, 35, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Struck, J.; Morgenthaler, N.G.; Bergmann, A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides 2005, 26, 2500–2504. [Google Scholar] [CrossRef]
- Palmiere, C.; Augsburger, M. Copeptin as a diagnostic biomarker for sepsis-related deaths. Peptides 2014, 59, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. Bench-to-bedside review: Vasopressin in the management of septic shock. Crit. Care 2011, 15, 226. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, N.G.; Struck, J.; Jochberger, S.; Dünser, M.W. Copeptin: Clinical use of a new biomarker. Trends Endocrinol. Metab. 2008, 19, 43–49. [Google Scholar] [CrossRef]
- Wilson, M.F.; Brackett, D.J.; Tompkins, P.; Benjamin, B.; Archer, L.T.; Hinshaw, L.B. Elevated plasma vasopressin concentrations during endotoxin and E coli. shock. Adv. Shock Res. 1981, 6, 15–26. [Google Scholar] [PubMed]
- Russell, J.A.; Walley, K.R.; Singer, J.; et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N. Engl. J. Med. 2008, 358, 877–887. [Google Scholar] [CrossRef]
- Latronico, N.; Castioni, C.A. Copeptin in critical illness. Clin. Chem. Lab. Med. 2014, 52, 1391–1393. [Google Scholar] [CrossRef]
- Barat, C.; Simpson, L.; Breslow, E. Properties of human vasopressin precursor constructs: Inefficient monomer folding in the absence of copeptin as a potential contributor to diabetes insipidus. Biochemistry 2004, 43, 8191–8203. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Müller, B.; Struck, J.; Bergmann, A.; Redl, H.; Christ-Crain, M. Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock 2007, 28, 219–226. [Google Scholar] [CrossRef]
- Ho, J.; Al-Musalhi, H.; Chapman, M.; et al. Septic shock and sepsis: A comparison of total and free plasma cortisol levels. J. Clin. Endocrinol. Metab. 2006, 91, 105–114. [Google Scholar] [CrossRef]
- Arafah, B.M. Hypothalamic pituitary adrenal function during critical illness: Limitations of current assessment methods. J. Clin. Endocrinol. Metab. 2006, 91, 3725–3745. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J.; Eichacker, P.Q.; Natanson, C. The effects of steroids during sepsis depend on dose and severity of illness: An updated meta-analysis. Clin. Microbiol. Infect. 2009, 15, 308–318. [Google Scholar] [CrossRef]
- Koh, G.C.K.W.; Peacock, S.J.; van der Poll, T.; Wiersinga, W.J. The impact of diabetes on the pathogenesis of sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 379–388. [Google Scholar]
- Levy, B. Lactate and shock state: The metabolic view. Curr. Opin. Crit. Care 2006, 12, 315–321. [Google Scholar] [CrossRef]
- Krinsley, J.S.; Fisher, M. The diabetes paradox: Diabetes is not independently associated with mortality in critically ill patients. Hosp. Pract. 2012, 40, 31–35. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Isaacs, S.D.; Bazargan, N.; You, X.; Thaler, L.M.; Kitabchi, A.E. Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. J. Clin. Endocrinol. Metab. 2002, 87, 978–982. [Google Scholar] [CrossRef]
- Brunkhorst, F.; Engel, C.; Bloos, F.; et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N. Engl. J. Med. 2008, 358, 125–139. [Google Scholar]
- NICE-SUGAR Study Investigators; Finfer, S.; Chittock, D.R.; et al. Intensive versus Conventional Glucose Control in Critically Ill Patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar]
- Devos, P.; Preiser, J.C.; Melot, C. Impact of tight glucose control by intensive insulin therapy on ICU mortality and the rate of hypoglycemia: Final results of the Glucontrol study. Intensive Care Med. 2007, 33, S189. [Google Scholar]
- Paz-Filho, G.; Mastronardi, C.; Franco, B.C.; Wang, K.B.; Wong, M.L.; Licinio, J. Leptin: Molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq. Bras. Endocrinol. Metab. 2012, 56, 597–607. [Google Scholar] [CrossRef]
- Shapiro, N.I.; Khankin, E.V.; Van Meurs, M.; et al. Leptin exacerbates sepsis-mediated morbidity and mortality. J. Imunol. 2010, 185, 517–524. [Google Scholar] [CrossRef]
- Behnes, M.; Brueckmann, M.; Lang, S.; et al. Alterations of leptin in the course of inflammation and severe sepsis. BMC Infect. Dis. 2012, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Weiskirchen, R.; Zimmermann, H.W.; Sanson, E.; Trautwein, C.; Tacke, F. Relevance of serum leptin and leptin-receptor concentrations in critically ill patients. Mediators Inflamm. 2010, 2010, 473540. [Google Scholar] [CrossRef] [PubMed]
- Lam, Q.L.; Lu, L. Role of leptin in immunity. Cell Mol. Immunol. 2007, 4, 1–13. [Google Scholar] [PubMed]
- Bracho-Riquelme, R.L.; Reyes-Romero, M.A. Leptin in sepsis: A well-suited biomarker in critically ill patients? Crit. Care 2010, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.A.; Amr, Y.M.; Suliman, G.A. The diagnostic value of serum leptin monitoring and its correlation with tumour necrosis factor-alpha in critically ill patients: A prospective observational study. Crit. Care 2010, 14, R33. [Google Scholar] [CrossRef]
- Sharma, S.; Dabla, P.K.; Kumar, S.; Dublis, S. Thyroid hormone dysfunction and CRP levels in neonates with sepsis. J. Endocrinol. Metab. 2013, 3, 62–66. [Google Scholar] [CrossRef]
- Angelousi, A.G.; Karageorgopoulos, D.E.; Kapaskelis, A.M.; Falagas, M.E. Association between thyroid function test at baseline and the outcome of patients with sepsis or septic shock: A systematic review. Eur. J. Endocrinol. 2011, 164, 147–155. [Google Scholar] [CrossRef]
- Zuppa, A.F.; Nadkarni, V.; Davis, L.; et al. The effect of a thyroid hormone infusion on vasopressor support in critically ill children with cessation of neurologic function. Crit. Care Med. 2004, 32, 2318–2322. [Google Scholar] [CrossRef] [PubMed]
| Hormone | The Type of Endocrine Response in Sepsis and Specific Eequirements |
|---|---|
| Vasopressin | Activation and depression of hypothalamic-pituitary-adrenal (HPA) axis with biphasic vasopressin secretory response and inappropriately low levels compared to the severity of illness; it is not recommended as first line vasopressor and should be used in low dose in addition to vasopressors (norepinephrine) |
| Copeptin | Copeptin concentration increases in parallel with the severity of the disease and accurately discriminates between patients with favorable and unfavorable outcomes; it reliably mirrors the production of arginine vasopressin, closely reflects the individual stress level compared to cortisol and is extremely stable in plasma or serum ex vivo |
| Cortisol | Activation and depression of HPA axis which leads to critical illness-related corticosteroid insufficiency; it is associated with poor outcome in sepsis; it is recommended to use intravenous hydrocortisone alone in patients with sepsis-induced hypotension refractory to fluid replacement and vasopressor therapy |
| Insulin | There is a tendency to hyperglycemia; it is a marker of severity of illness and a predictor of poor outcome in patients without previous diabetes; a tight glycemic control is not recommended |
| Leptin | It increases early during sepsis and correlates with sepsis severity; it could play a role in the pathogenesis of sepsis-associated multi-organ failure through pro-inflammatory actions |
| Thyroid hormones | Depression of thyroid function is generally a transient event, but it seems to be associated with a worse prognosis; it does not require specific treatment |
© GERMS 2015.
Share and Cite
Gheorghiță, V.; Barbu, A.E.; Gheorghiu, M.L.; Căruntu, F.A. Endocrine Dysfunction in Sepsis: A Beneficial or Deleterious Host Response? Germs 2015, 5, 17-25. https://doi.org/10.11599/germs.2015.1067
Gheorghiță V, Barbu AE, Gheorghiu ML, Căruntu FA. Endocrine Dysfunction in Sepsis: A Beneficial or Deleterious Host Response? Germs. 2015; 5(1):17-25. https://doi.org/10.11599/germs.2015.1067
Chicago/Turabian StyleGheorghiță, Valeriu, Alina Elena Barbu, Monica Livia Gheorghiu, and Florin Alexandru Căruntu. 2015. "Endocrine Dysfunction in Sepsis: A Beneficial or Deleterious Host Response?" Germs 5, no. 1: 17-25. https://doi.org/10.11599/germs.2015.1067
APA StyleGheorghiță, V., Barbu, A. E., Gheorghiu, M. L., & Căruntu, F. A. (2015). Endocrine Dysfunction in Sepsis: A Beneficial or Deleterious Host Response? Germs, 5(1), 17-25. https://doi.org/10.11599/germs.2015.1067




