Disseminated Tuberculosis in HIV-Infected Patients from the Regional HIV/AIDS Center Constanța, Romania
Abstract
Introduction
Methods
Statistical Analysis
Results
Discussion
Conclusion
Conflicts of interest
References
- A.D.A.M. Medical Encyclopedia. Atlanta (GA): A.D.A.M., Inc.; 2012. Disseminated tuberculosis. Accessed on: January 03, 2014. Available online: http://www.nlm.nih.gov/medlineplus/ency/article/000 624.htm.
- HIV InSite. Tuberculosis and HIV. Available online: http://hivinsite.ucsf.edu/insite?page=kb-05-01-06 (accessed on 3 January 2014).
- World Health Organization (WHO), TB/HIV Facts 2011/12. Available online: http://www.who.int/tb/publications/TBHIV_Facts_for_2011.pdf (accessed on 3 January 2014).
- Kamath, R.; Sharma, V.; Pattanshetty, S.; Hegde, M.B.; Chandrasekaran, V. HIV-TB coinfection: Clinico- epidemiological determinants at an antiretroviral therapy center in Southern India. Lung India. 2013, 30, 302–6. [Google Scholar] [PubMed]
- Gao, L.; Zhou, F.; Li, X.; Jin, Q. HIV/TB co-infection in mainland China: a meta-analysis. PLoS One. 2010, 5, e10736. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Mohan, A.; Kadhiravan, T. HIV-TB co- infection: epidemiology, diagnosis & management. Indian J Med Res. 2005, 121, 550–67. [Google Scholar] [PubMed]
- Klatt, E.C. Pathology of AIDS. Version 24. Savannah: Mercer University School of Medicine. 2013. [Google Scholar]
- Leeds, I.L.; Magee, M.J.; Kurbatova, E.V.; et al. Site of extrapulmonary tuberculosis is associated with HIV infection. Clin Infect Dis. 2012, 55, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.E.; Young, S.M.; Antoniskis, D.; Davidson, P.T.; Kramer, F.; Barnes, P.F. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993, 148, 1292–7. [Google Scholar] [CrossRef] [PubMed]
- Affusim, C.C.; Kesieme, E.; Abah, V.O. The pattern of presentation and prevalence of tuberculosis in HIV- seropositive patients seen at Benin City, Nigeria. ISRN Pulmonology. 2012, 2012, 6–12. [Google Scholar] [CrossRef]
- Vajpayee, M.; Kanswal, S.; Seth, P.; Wig, N.; Pandey, R.M. Tuberculosis infections in HIV-infected Indian patients. AIDS Patient Care STDS. 2004, 18, 209–13. [Google Scholar] [CrossRef] [PubMed]
- Jaryal, A.; Raina, R.; Sarkar, M.; Sharma, A. Manifestations of tuberculosis in HIV/AIDS patients and its relationship with CD4 count. Lung India. 2011, 28, 263–6. [Google Scholar] [PubMed]
- von Reyn, C.F.; Kimambo, S.; Mtei, L.; et al. Disseminated tuberculosis in human immunodeficiency virus infection: ineffective immunity, polyclonal disease and high mortality. Int J Tuberc Lung Dis. 2011, 15, 1087–92. [Google Scholar] [CrossRef] [PubMed]
- Chamie, G.; Luetkemeyer, A.; Walusimbi-Nanteza, M.; et al. Significant variation in presentation of pulmonary tuberculosis across a high resolution of CD4 strata. Int J Tuberc Lung Dis. 2010, 14, 1295–302. [Google Scholar] [PubMed]
- El-Hazmi, M.M.; Al-Otaibi, F.E. Predictors of pulmonary involvement in patients with extra-pulmonary tuberculosis. J Family Community Med. 2012, 19, 88–92. [Google Scholar] [PubMed]
- Iredia, C.H.; Oguntibeju, O.O.; Lewis, H.A.; Mokwena, K. Trends and characteristics of patients admitted with musculoskeletal tuberculosis to a referral hospital from 2003 to 2008. Afr J Microbiol Res. 2011, 5, 532–40. [Google Scholar]
- Nancoz, O.; Kherad, O.; Perrin, E.; Hsu, C.; Lobrinus, J.A.; Nendaz, M. Disseminated tuberculosis presenting with polymorphonuclear effusion and septic shock in an HIV- seropositive patient: a case report. J Med Case Rep. 2010, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Abdi-Liae, Z.; Moradnejad, P.; Alijani, N.; Khazraiyan, H.; Mansoori, S.; Mohammadi, N. Disseminated tuberculosis in an AIDS/HIV-infected patient. Acta Med Iran. 2013, 51, 587–9. [Google Scholar] [PubMed]
- Ntsekhe, M.; Mayosi, B.M. Tuberculous pericarditis with and without HIV. Heart Fail Rev. 2013, 18, 367–73. [Google Scholar] [CrossRef] [PubMed]
- Oni, T.; Burke, R.; Tsekela, R.; et al. High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening. Thorax. 2011, 66, 669–73. [Google Scholar] [CrossRef] [PubMed]
- Mtei, L.; Matee, M.; Herfort, O.; et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005, 40, 1500–7. [Google Scholar] [CrossRef] [PubMed]
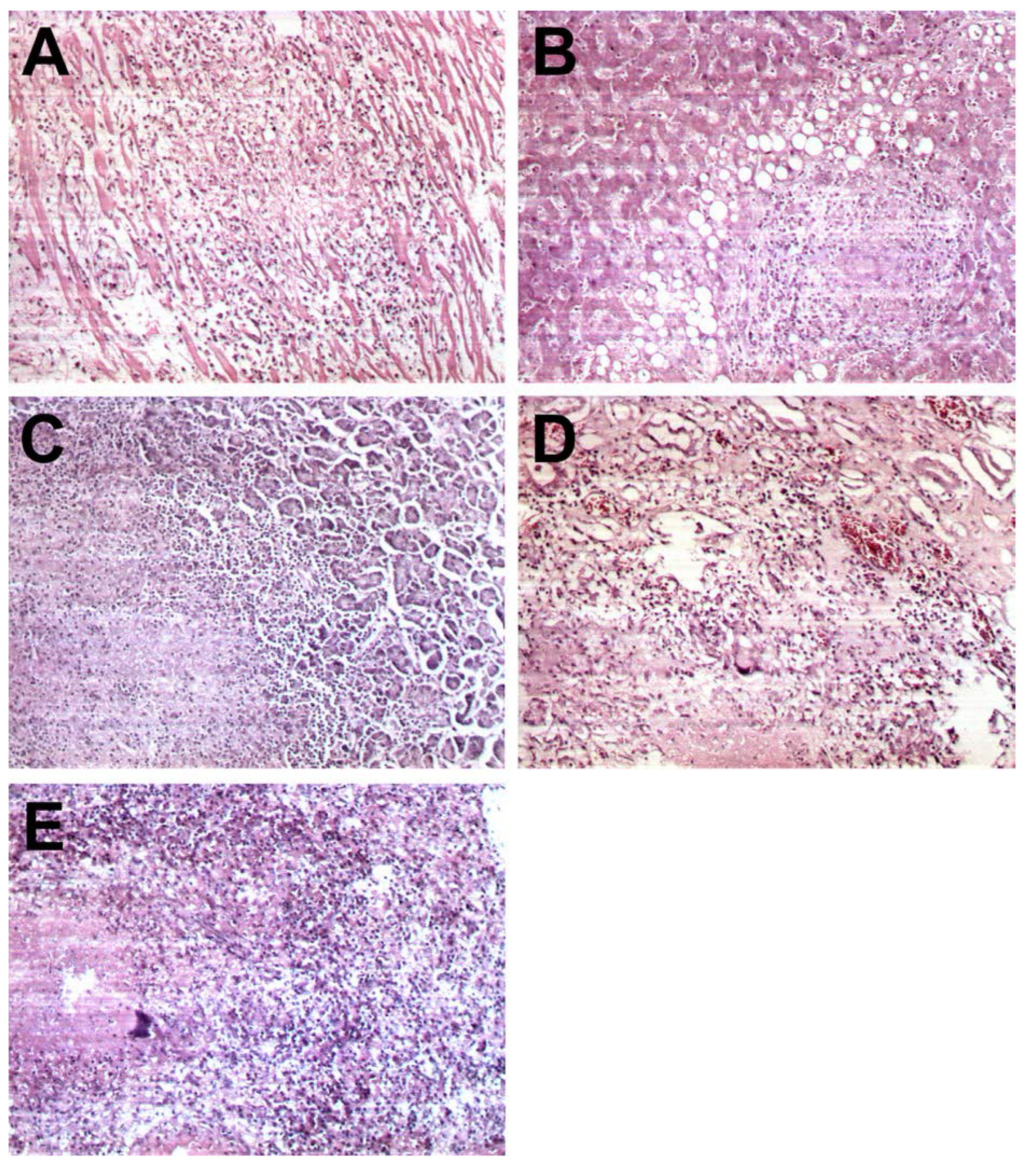
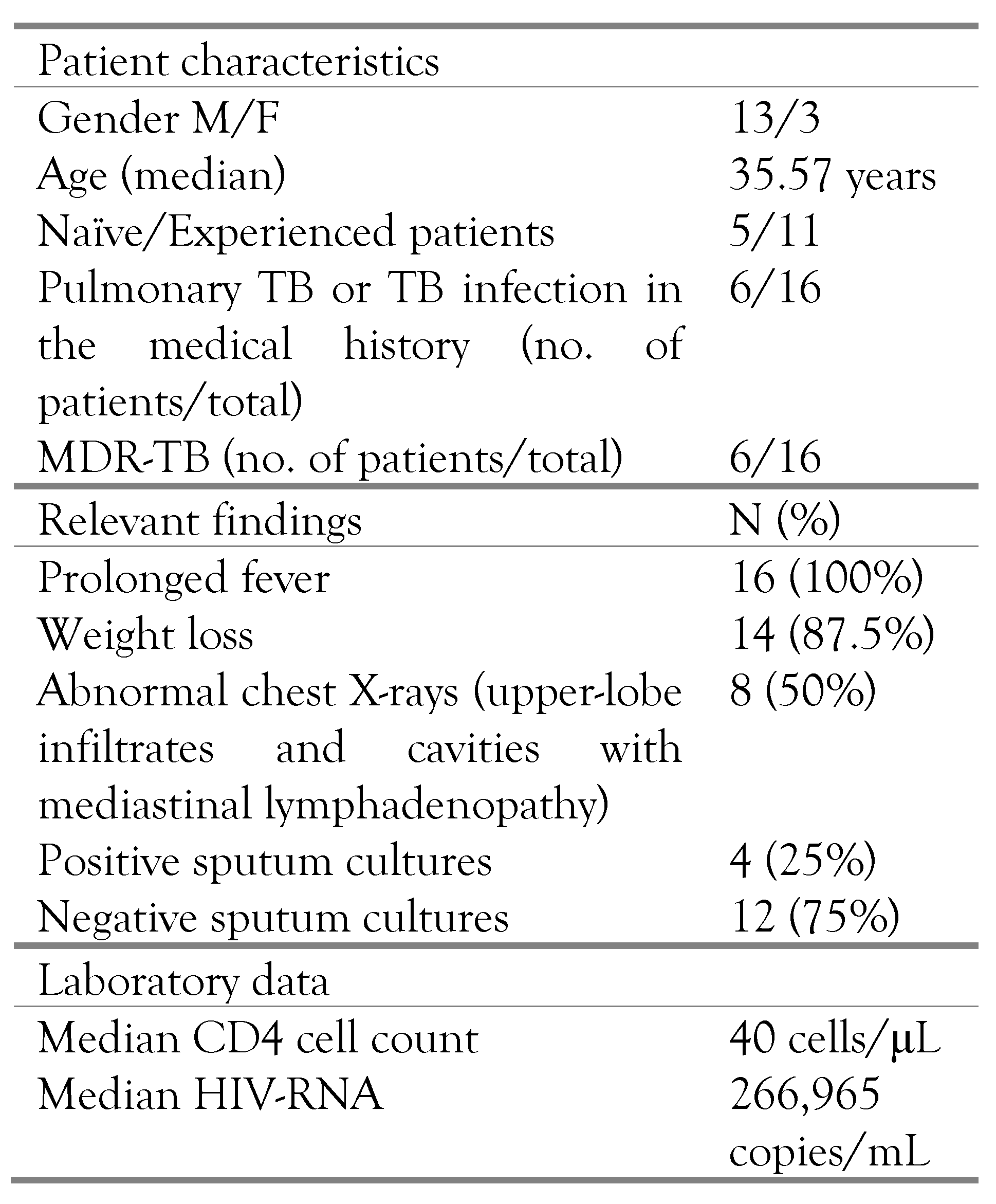
| Pathological examination –organ involvement | N (%) |
|---|---|
| Lung | 12 (75%) |
| Mediastinal lymphadenopathy | 11 (68.75%) |
| Liver | 2 (12.5%) |
| Spleen | 4 (25%) |
| Kidney | 4 (25%) |
| Pancreas | 1 (6.25%) |
| Meninges | 3 (18.75%) |
| Pleura | 5 (31.25%) |
| Pericardium | 3 (18.75%) |
| Heart | 3 (18.75%) |
| Peritoneum | 5 (31.25%) |
© GERMS 2014.
Share and Cite
Rugină, S.; Dumitru, I.-M.; Resul, G.; Cernat, R.C.; Petcu, A.E. Disseminated Tuberculosis in HIV-Infected Patients from the Regional HIV/AIDS Center Constanța, Romania. GERMS 2014, 4, 16-21. https://doi.org/10.11599/germs.2014.1050
Rugină S, Dumitru I-M, Resul G, Cernat RC, Petcu AE. Disseminated Tuberculosis in HIV-Infected Patients from the Regional HIV/AIDS Center Constanța, Romania. GERMS. 2014; 4(1):16-21. https://doi.org/10.11599/germs.2014.1050
Chicago/Turabian StyleRugină, Sorin, Irina-Magdalena Dumitru, Ghiulendan Resul, Roxana Carmen Cernat, and Andra Elena Petcu. 2014. "Disseminated Tuberculosis in HIV-Infected Patients from the Regional HIV/AIDS Center Constanța, Romania" GERMS 4, no. 1: 16-21. https://doi.org/10.11599/germs.2014.1050
APA StyleRugină, S., Dumitru, I.-M., Resul, G., Cernat, R. C., & Petcu, A. E. (2014). Disseminated Tuberculosis in HIV-Infected Patients from the Regional HIV/AIDS Center Constanța, Romania. GERMS, 4(1), 16-21. https://doi.org/10.11599/germs.2014.1050




