Cardiac Involvement in HIV-Positive Patients
Abstract
Introduction
Methods
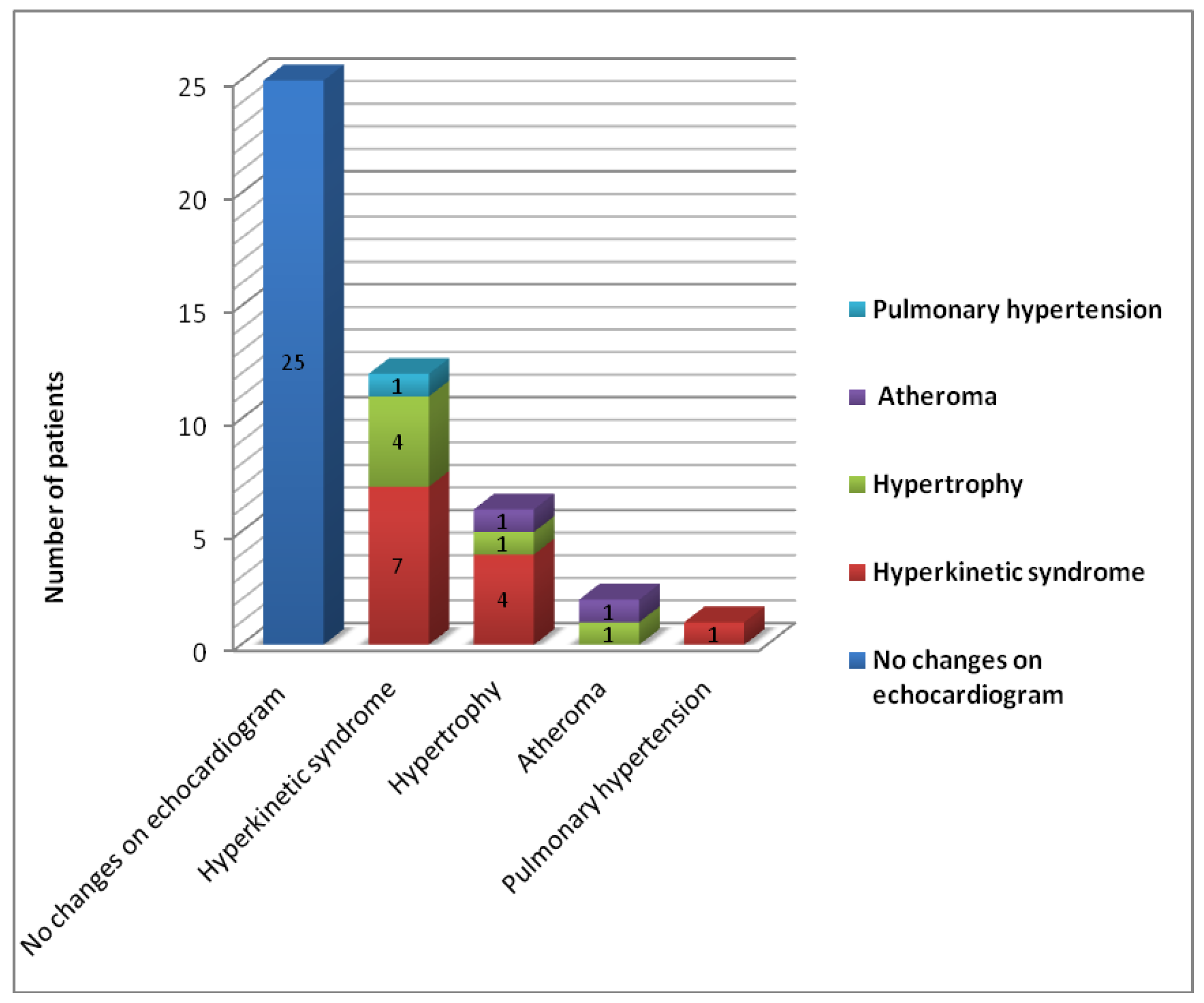
Results
Discussion
Conflicts of Interest
Author Contributions
References
- UNAIDS Report on the global AIDS epidemic 2012. Accessed on: December 29, 2012. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_en.pdf.
- Dragović, G.; Jevtović, D. Highlights from the 13th European AIDS Conference (EACS). GERMS 2011, 1, 9–11. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS World AIDS Day Report 2012. Accessed on: December 29, 2012. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/JC2434_WorldAIDSday_results_en.pdf.
- Compartment for monitoring and evaluating HIV/AIDS infection in Romania. National Institute for Infectious Diseases “Prof.Dr. Matei Balş”. HIV/AIDS infection at December 1, 2012. Accessed on: December 17, 2012. Available at: http://www.cnlas.ro/images/doc/1dec2012.pdf.
- Cheitlin, M.D. Cardiac involvement in HIV-infected patients. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2013.
- May, M.T.; Sterne, J.A.; Costagliola, D.; Sabin, C.A.; Phillips, A.N.; Justice, A.C.; et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: A collaborative analysis. Lancet 2006, 368, 451–458. [Google Scholar] [CrossRef]
- Barbaro, G. Immune dysfunction and immunotherapy in heart disease. In: Watson R, Larson D, (Ed), Blackwell Futura; 2007.
- Barbaro, G.; Barbarini, G. Human immunodeficiency virus & cardiovascular risk. Indian J Med Res. 2011, 134, 898–903. [Google Scholar] [CrossRef]
- Streinu-Cercel, A.; Ion, D.A.; Chivu, L.I.; Chivu, R.D. Lipodystrophy syndrome in HIV-infected patients. Clinical and diagnostic features. Rev Med Chir Soc Med Nat Iasi. 2006, 110, 521–525. [Google Scholar] [PubMed]
- Guaraldi, G. Evolving approaches and resources for clinical practice in the management of HIV infection in the HAART era. GERMS 2011, 1, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, M.; Jevtović, D.; Dragović, G. Predicting HIV treatment response in Romania—Comment. GERMS 2012, 2, 23–24. [Google Scholar] [CrossRef]
- Revell, A.; Ene, L.; Duiculescu, D.; Wang, D.; Youle, M.; Pozniak, A.; et al. The use of computational models to predict response to HIV therapy for clinical cases in Romania. GERMS 2012, 2, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Revell, A.; Wang, D.; Alvarez-Uria, G.; Streinu-Cercel, A.; Ene, L.; Wensing, A.; et al. Computational models that predict response to HIV therapy can reduce virological failure and therapy costs in resource-limited settings. J Int AIDS Soc. 2012, 15, 18114. [Google Scholar] [CrossRef]
- Aramă, V.; Tilişcan, C.; Ion, D.; Mihăilescu, R.; Munteanu, D.; Streinu-Cercel, A.; et al. Serum adipokines and HIV viral replication in patients undergoing antiretroviral therapy. GERMS 2012, 2, 12–17. [Google Scholar] [CrossRef]
- Longo, D.; Fauci, A.; Kasper, D.; Hauser, S.; Jameson, J.; Loscalzo, J. Harrison’s Principles of Internal Medicine, 18th Edition. Chapter 234. Heart Failure and Cor Pulmonale McGraw-Hill; 2011.
- Streinu-Cercel, O. AIDS and Sexually Transmitted Infections in Africa. GERMS 2012, 2, 5. [Google Scholar] [CrossRef]
- Streinu-Cercel, A.; de Gorgolas, M.; Muller, M.; Portilla, J.; Rugina, S.; Bocher, W.; et al. Switching from a toxicity-causing antiretroviral to enfuvirtide in patients with HIV: The SWITCH TOX study. HIV Clin Trials. 2008, 9, 375–386. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Herskowitz, A.; Vlahov, D.; Willoughby, S.; et al. Prevalence and incidence of left ventricular dysfunction in patients with human immunodeficiency virus infection. Am J Cardiol 1993, 71, 955–958. [Google Scholar] [CrossRef]
- Barbaro, G.; Di Lorenzo, G.; Grisorio, B.; Barbarini, G. Incidence of dilated cardiomyopathy and detection of HIV in myocardial cells of HIV-positive patients. Gruppo Italiano per lo Studio Cardiologico dei Pazienti Affetti da AIDS. N Engl J Med. 1998, 339, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Rabinstein, A.A.; Elkind, M.S.; Powers, W.J. Recent developments regarding human immunodeficiency virus infection and stroke. Cerebrovasc Dis. 2012, 33, 209–218. [Google Scholar] [CrossRef]
- Lopez, M.; Vispo, E.; San Roman, J.; Herrero, D.; Peris, A.; Corral, A.; et al. Short communication high risk of endothelial dysfunction in HIV individuals may result from deregulation of circulating endothelial cells and endothelial progenitor cells. AIDS Res Hum Retroviruses 2012, 28, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Osakwe, C.E.; Bleotu, C.; Chifiriuc, M.C.; Grancea, C.; Otelea, D.; Paraschiv, S.; et al. TH1/TH2 cytokine levels as an indicator for disease progression in human immunodeficiency virus type 1 infection and response to antiretroviral therapy. Roum Arch Microbiol Immunol. 2010, 69, 24–34. [Google Scholar] [PubMed]
- Streinu-Cercel, A. Nanoparticles. GERMS 2012, 2, 90. [Google Scholar] [CrossRef]
- Helleberg, M.; Afzal, S.; Kronborg, G.; Larsen, C.S.; Pedersen, G.; Pedersen, C.; et al. Mortality Attributable to Smoking Among HIV-1-Infected Individuals: A Nationwide, Population-Based Cohort Study. Clin Infect Dis. 2012. [Google Scholar] [CrossRef] [PubMed]
- WHO Report on the global tobacco epidemic, 2011. Warning about the dangers of tobacco. Accessed on: , 2012. Available at: http://whqlibdoc.who.int/publications/2011/9789240687813_eng.pdf.
- Tesoriero, J.M.; Gieryic, S.M.; Carrascal, A.; Lavigne, H.E. Smoking among HIV positive New Yorkers: Prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010, 14, 824–835. [Google Scholar] [CrossRef]
- Madeddu, G.; Fois, A.G.; Calia, G.M.; Babudieri, S.; Soddu, V.; Becciu, F.; et al. Chronic obstructive pulmonary disease: An emerging comorbidity in HIV-infected patients in the HAART era? Infection 2012. [Google Scholar] [CrossRef] [PubMed]
- Wewers, M.D.; Diaz, P.T.; Wewers, M.E.; Lowe, M.P.; Nagaraja, H.N.; Clanton, T.L. Cigarette smoking in HIV infection induces a suppressive inflammatory environment in the lung. Am J Respir Crit Care Med. 1998, 158 Pt 1, 1543–1549. [Google Scholar] [CrossRef]
- Oliviero, U.; Bonadies, G.; Bosso, G.; Foggia, M.; Apuzzi, V.; Cotugno, M.; et al. Impaired diastolic function in naive untreated human immunodeficiency virus infected patients. World J Cardiol. 2010, 2, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Sabin, C.A.; Worm, S.W.; Weber, R.; Reiss, P.; El-Sadr, W.; Dabis, F.; et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: A multi- cohort collaboration. Lancet 2008, 371, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Interpretation | WCA (n = 15) | WOCA (n = 25) | All patients (n = 40) |
| Gender | Female | 6 | 17 | 23 |
| Male | 9 | 8 | 17 | |
| Age | <30 | 11 | 18 | 29 |
| >30 | 4 | 7 | 11 | |
| Cholesterol | Normal | 9 | 20 | 29 |
| High | 6 | 5 | 11 | |
| LDL | Optimal | 7 | 14 | 21 |
| Borderline | 4 | 6 | 10 | |
| High | 4 | 5 | 9 | |
| HDL | Low | 3 | 5 | 8 |
| Normal | 6 | 12 | 18 | |
| High | 6 | 8 | 14 | |
| TG | Normal | 11 | 20 | 31 |
| High | 4 | 5 | 9 | |
| Serum glucose | <100 | 15 | 25 | 40 |
| HbA1c | 4–5.9 | 15 | 25 | 40 |
| Blood pressure | <120/80 | 15 | 25 | 40 |
| Framingham | At risk (above 10%) | 1 | 1 | 2 |
| score | Not at significant risk | 14 | 24 | 38 |
| (under 10%) | ||||
| Smoking | Yes (10 pack-years) | 11 | 13 | 24 |
| No (below 10 pack- | 4 | 12 | 16 | |
| years) | ||||
| Alcohol use | Yes | 7 | 14 | 21 |
| No | 8 | 11 | 19 | |
| Time from | 10–14 years | 2 | 2 | 4 |
| infection | 15–20 years | 1 | 4 | 5 |
| 21–25 years | 12 | 19 | 31 | |
| Duration of | 10–14 years | 3 | 2 | 5 |
| treatment | 15–20 years | 12 | 23 | 35 |
| Treatment | NNRTI | 3 | 1 | 4 |
| regimen | NNRTI/PI | 12 | 24 | 36 |
| Risk factor | Odds Ratio | 90%CI |
| Cholesterol AND smoking | 5.75 | 1.263–26.169 |
| Gender (male) | 3.187 | 1.038–9.779 |
| Cholesterol (>200 mg/dL) | 2.666 | 0.804–8.842 |
| Smoking (above 10 packs-year) | 2.538 | 0.789–8.163 |
| Low HDL (<40 mg/dL) | 1.416 | 0.461–4.346 |
| High LDL (>130 mg/dL) | 1.454 | 0.409–5.170 |
| High TG (>200 mg/dL) | 1.454 | 0.409–5.170 |
| Age >30 years | 0.935 | 0.278–3.141 |
| Alcohol consumption | 0.687 | 0.232–2.028 |
| Duration of ARV therapy (>15 years) | 0.347 | 0.069–1.751 |
© GERMS 2013.
Share and Cite
Daglan, E.; Yamin, D.; Manu, B.; Streinu-Cercel, A. Cardiac Involvement in HIV-Positive Patients. GERMS 2013, 3, 8-13. https://doi.org/10.11599/germs.2013.1031
Daglan E, Yamin D, Manu B, Streinu-Cercel A. Cardiac Involvement in HIV-Positive Patients. GERMS. 2013; 3(1):8-13. https://doi.org/10.11599/germs.2013.1031
Chicago/Turabian StyleDaglan, Efrat, Dan Yamin, Bogdana Manu, and Anca Streinu-Cercel. 2013. "Cardiac Involvement in HIV-Positive Patients" GERMS 3, no. 1: 8-13. https://doi.org/10.11599/germs.2013.1031
APA StyleDaglan, E., Yamin, D., Manu, B., & Streinu-Cercel, A. (2013). Cardiac Involvement in HIV-Positive Patients. GERMS, 3(1), 8-13. https://doi.org/10.11599/germs.2013.1031




