Introduction
In 1912, Klinger isolated a coccobacillus from human actinomycotic lesions which he named
Actinobacillus actinomycetemcomitans; in 2006, following genetic studies, it was reclassified with its current name [
1,
2].
Aggregatibacter actinomycetemcomitans is a facultatively anaerobic, capnophile, non-sporulated, immobile Gram-negative rod commensal in the human oral cavity of 25-30% of healthy adults [
2]. Its presence in the oral cavity is associated with cavities, periodontitis, and other dental pathologies [
1,
2]. It can represent the source of local or systemic infections with serious evolution, in particular infective endocarditis. Due to multiple virulence factors, such as the ability to form biofilm (due to fimbriae that facilitate adhesion to host surfaces), it represents a challenge in the treatment of systemic infections, especially in patients with valve prostheses or valvulopathies [
1,
2,
3]. Complete eradication of
Aggregatibacter actinomycetemcomitans infection requires prolonged antibiotic therapy, the antibiotic of choice being ceftriaxone. It is recommended to administer the treatment for at least 4 weeks, depending on the patient's clinical response, as well as solving dental problems to prevent possible recurrences [
2,
4].
We present a particular case of an adult male patient diagnosed with infective endocarditis with A. actinomycetemcomitans and patent ductus arteriosus (PDA) treated in the National Institute of Infectious Diseases "Prof. Dr. Matei Balș" from Bucharest, Romania.
Case report
A 37-year-old male patient with a history of chronic ethanol consumption was admitted on the third week of the disease with low-grade fever (37.2°C), a cough initially dry, later with mucopurulent sputum, stabbing pain in the posterior lower left thoracic half, headache and dizziness. The clinical examination revealed poor dental hygiene with numerous cavities and missing teeth, a week-old skin rash distally on the calves, tachycardia (92 bpm), hypotension (100/60 mmHg), a pansystolic heart murmur of 5/6 intensity audible in all auscultation areas, liver of hard consistency palpable approximately 3 cm below the costal edge, vesicular murmur slightly decreased on the left side, and an O2 peripheral saturation of 96% in atmospheric air.
Laboratory test results were as follows: leukocytosis – 21,600/µL, neutrophilia – 78%, a high C-reactive protein (CRP) of 93.5 mg/L, an elevated procalcitonin level of 1.03 ng/mL, hemoglobin – 6.7g/dL, prothrombin time (PT) – 20.9 sec, International Normalized Ratio (INR) – 1.76, glycemia – 127 mg/dL (
Table 1).
A lung infection was suspected and a chest X-ray was performed, describing mixed hiliobasal pneumonia on the left side, a marked peribronchovascular pattern on a fibrous background, and left lower interlobar cystic pleurisy. Thus, empiric antibiotic treatment was started with amoxicillin/clavulanic acid (1.2 g every 8 hours) and doxycycline (100 mg every 12 hours). On the same day, a set of blood cultures collected while febrile and a sputum sample were sent to the bacteriology laboratory.
The sputum sample was positive on day 2 with a susceptible strain of Klebsiella pneumoniae and the antibiotic therapy was switched to piperacillin-tazobactam (4.5 g every 6 hours).
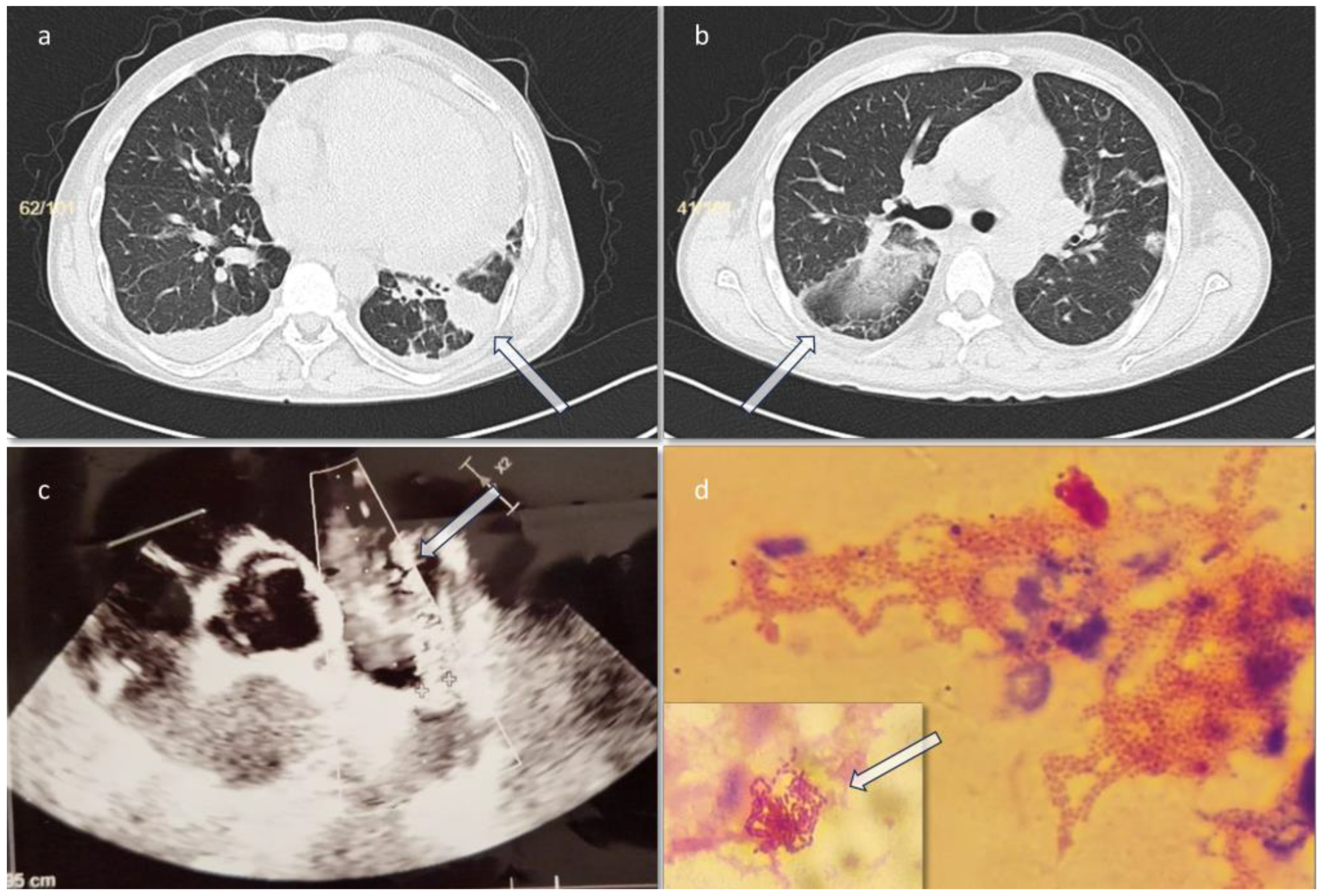
On day 3 he presented an episode of sinus tachycardia. A cardiology consult and a transthoracic sonography were performed, showing a filamentous formation of 1 cm attached to the pulmonary valve, suggestive for valve vegetations, and a persistent ductus arteriosus (PDA) (
Figure 1). The left ventricular ejection fraction (LVEF) was 45%. Treatment with metoprolol (50 mg every 24 hours) was initiated and a suspicion of infective endocarditis was raised. In this context, the antibiotic treatment started with piperacillin-tazobactam was continued, and vancomycin (1 g every 12 hours) was added to cover the possibility of Gram-positive cocci involvement.
Chest CT scan performed on the third day of admission revealed two filling defects of approximately 12 mm and 10 mm respectively, at the level of the pulmonary artery trunk – possibly thrombotic images/vegetation/mediastinal adenopathies, for which deep vein thrombosis prophylaxis with enoxaparin (0.6 mL every 12h for 14 days then 0.6 mL every 24h for another 7 days) was initiated (as the cardiologist recommended), an inhomogeneous image of approximately 6/3.5 cm at the level of the left lung lower lobe, posterior and lateral segments, with mixed densities (liquid and air) suggestive of a partially evacuated lung abscess (
Figure 1a, 1b). On day 4 the result for blood cultures was positive with
A. actinomycetemcomitans. Subsequently, the antibiotic treatment was deescalated to ceftriaxone (2 g/day), to which both A.
actinomycetemcomitans and
K. pneumoniae were susceptible.
After 3 days of monotherapy with ceftriaxone (and on 8th day of admission) the patient remained subfebrile, and the biological samples showed the reappearance of leukocytosis with neutrophilia (84%) and a significant inflammatory syndrome (CRP 144 mg/L, almost two times higher than the moment of admission). Another possible associated germ (probably of pulmonary etiology) was considered and a narrower spectrum antibiotic, active on such a germ - gentamicin (80 mg every 12 hours, IV) for 7 days, was added to the treatment regimen. Under this treatment, the leukocyte count returned to normal values, the inflammatory syndrome decreased, and the clinical state improved with normal temperature levels. We concluded that most probably another germ was involved, leading to the previous biological changes.
Intravenous antibiotic treatment was continued with ceftriaxone 2 g/day only. The biologic evolution was good, but productive cough and thoracic pain persisted. It was decided together with the pulmonologist to perform bronchoscopy and alveolar lavage at the end of the third week of hospitalization. Vancomycin-resistant Enterococcus faecium (VRE) was isolated from the broncho-alveolar lavage and linezolid (600 mg every 12 hours) was added to the treatment for the next 18 days. Under this treatment the evolution was favorable. Since the persistence of ductus arteriosus was identified, the opinion of a cardiac surgery specialist was sought to determine whether surgery to close the congenital heart defect was necessary. It was decided to postpone the consultation and surgery after elimination of the infectious foci.
The clinical evolution under treatment was slowly favorable, with remission of cough, headache and dizziness. The patient was discharged upon request after 44 days of hospitalization. He was discharged afebrile, in good clinical condition (blood pressure 110/60 mmHg, ventricular rate 94/min, O2 peripheral saturation in atmospheric air 99%), with the recommendation to continue antibiotic therapy per os with amoxicillin/clavulanic acid (1 g every 24 hours) (thinking about the risk of other possible unidentified pulmonary germs to a person who can’t be monitored anymore) and linezolid 600 mg every 12 hours (for Enterococcus faecium VRE) for 7 days. Control imaging investigations (contrast CT scans) were performed every 10 days during the hospitalization. The final ultrasound no longer visualized pulmonary artery trunk vegetations. Note that at the first CT scan the dimensions of the pulmonary abscess in the left lower lobe were 6/3.5 cm and at the last CT scan the dimensions decreased to 4.4/2.7 cm. The two filling defects of 12 mm and 10 mm in the pulmonary artery trunk (possible thrombotic images/vegetation) were no longer visualized on CT after the first 10 days of treatment. Pulmonary thromboembolism of the lateral segment of the left lower lobar pulmonary artery was no longer seen. He was recalled for hospitalization and continuation of antibiotic therapy with intravenous ceftriaxone.
The patient returned after 10 days of oral therapy at home. On the CT examination, a favorable evolution was observed, the pulmonary abscess was in dimensional regression, with minimal fibrotic changes. On the transthoracic ultrasound, a 5 mm formation was visualized on the pulmonary valve. Treatment with ceftriaxone (2 g every 24 hours) was restarted for 16 days. The patient refused any surgical intervention for the heart defect. After completing a total of 60 days of treatment with ceftriaxone (2 g every 24h), he was discharged with improved biological and clinical parameters. A 14-day follow-up appointment was made, but he did not honor it.
Microbiological diagnosis
Two hemoculture sets (aerobic and anaerobic vials) were sent to the microbiology laboratory in the first 24 hours from admission. The anaerobic bottle became positive after 28 hours of incubation in the automatic BacT/Alert 3D system (BioMérieux, France). Gram-negative rods were observed isolated and in clusters (
Figure 1d) on the Gram stain. After 48 hours at 35±2°C, bacterial growth was obtained in anaerobiosis, characterized by small colonies in diameter (0.5-1.0 mm), white-grey in color, and non-hemolytic. The MALDI-TOF automated matrix-assisted laser desorption ionization (Bruker, USA) was used for identification, resulting in
Aggregatibacter actinomycetemcomitans. Antibiotic susceptibility was tested using E-tests, and the EUCAST version 13.0 standard (January 2023) was used for minimum inhibitory concentration (MIC) analysis. The strain was susceptible to all antibiotics tested.
Discussion
The incidence of infective endocarditis varies between 1.4 and 12.7 cases/100,000 people/year, having high morbidity and mortality rates and in most cases, the microbial agent is a Gram-positive bacterium [
2,
4]. However, the HACEK group -
Haemophilus spp.,
Aggregatibacter spp. (
A. actinomycetemcomitans, A. aphrophilus, A. paraphrophilus, and
A. segnis),
Cardiobacterium spp.,
Eikenella corrodens and
Kingella spp., fastidious Gram-negative bacteria, is responsible for approximately 1-3% of cases [
4]. The mortality of HACEK-associated infectious endocarditis is 3%, but they can be the source of major complications, such as emboli, heart failure or encephalitis [
4]. This group of bacteria is commensal to the oropharyngeal biota [
4,
5].
A. actinomycetemcomitans is the most common pathogen found in infectious endocarditis caused by the HACEK group [
1,
4]. HACEK endocarditis is usually characterized by an insidious evolution, non-specific symptoms, and an average duration since the onset of symptoms to diagnosis of up to 3 months for
Aggregatibacter spp [
4,
6]. The particularities of the case are the rapid microbiological diagnosis, after approximately 4 weeks since the onset of symptoms, and the fact that the patient presented PDA at an advanced age (37 years). PDA is a known risk factor for infective endocarditis, but with a low incidence [
7,
8].
Virulence factors are the main components of bacterial pathogenicity. They enable bacteria to colonize host tissues, evade host immune responses and cause damage locally or systemically. Regarding
Aggregatibacter actinomycetemcomitans, the most studied virulence factor is a protein called exotoxin called leukotoxin (LtxA) [
9,
10]. It was believed that leukotoxin targets only leukocytes, but recent studies proved that it can target many different cells such as monocytes, erythrocytes or endothelial cells [
10]. Initially, LtxA was thought to form pores in the membranes of leukocytes, leading to cell lysis and impairment of the immune response, but the studies have been inconclusive [
10]. Another virulence factor, cytolethal distending toxin (CDT) is a genotoxin and can cause DNA damage, with
A. actinomycetemcomitans being the only known oral species that can produce this toxin [
3]. One more particularity that increases the virulence potency of this organism is the lipopolysaccharide, specific to Gram-negative bacteria. The lipopolysaccharide upregulates the expression of microRNA miR-146a, which in turn downregulates the TNF receptor-associated factor 6 and IL-1 receptor-associated kinase 1, which functions as a negative feedback loop in cytokine signaling [
3].
Conclusions
Infective endocarditis caused by A. actinomycetemcomitans is a pathology that should be considered in patients with altered general condition and congenital heart defects. In the present case, the patient presented two risk factors, namely poor dental hygiene and patent ductus arteriosus.