Continuous Versus Intermittent Infusion of Beta-Lactam Antibiotics: Where Do We Stand Today? A Narrative Review
Abstract
Introduction
Methods
Search criteria
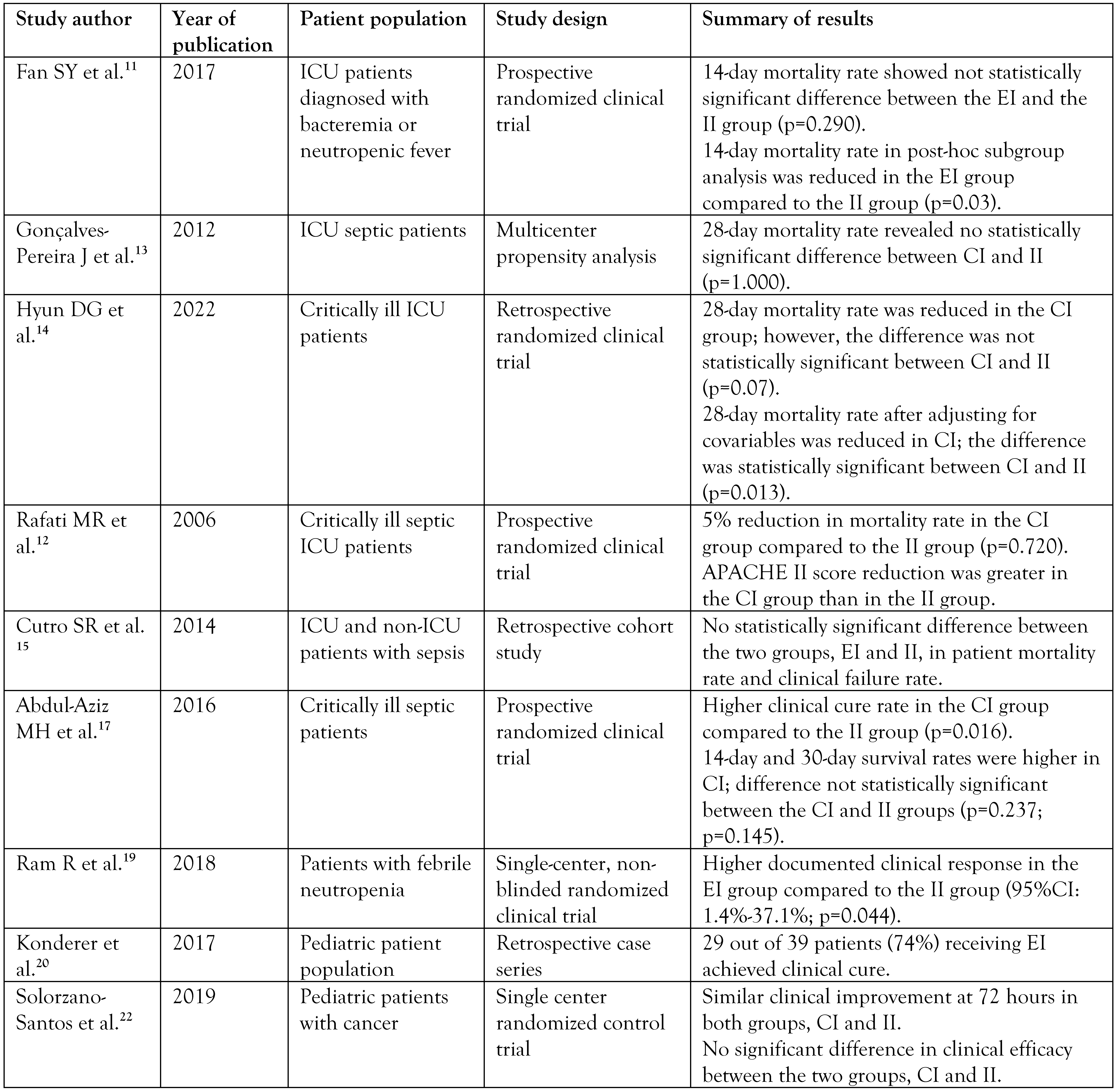
Results
Piperacillin-tazobactam
Critically ill patients
Febrile neutropenia
Pediatric patients
Cefepime
Critically ill patients
Febrile neutropenia
Pediatric patients
Meropenem
Critically ill patients
Febrile neutropenia
Pediatric patients
Discussion
Conclusions
Funding
Conflicts of Interest
References
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistenceImplications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Brunetti, L.; Poustchi, S.; Cunningham, D.; et al. Clinical and economic impact of empirical extended-infusion piperacillin-tazobactam in a community medical center. Ann. Pharmacother. 2015, 49, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.N.; Mynatt, R.P.; Kaye, K.S.; Zhao, J.J.; Pogue, J.M. Clinical outcomes with extended versus intermittent infusion of anti-pseudomonal beta-lactams in patients with gram-negative bacteremia. Open Forum Infect Dis. 2023, 10, ofad170. [Google Scholar] [CrossRef]
- Keng, M.K.; Sekeres, M.A. Febrile neutropenia in hematologic malignancies. Curr. Hematol. Malig. Rep. 2013, 8, 370–378. [Google Scholar] [CrossRef]
- Cotner, S.E.; Rutter, W.C.; Burgess, D.R.; Wallace, K.L.; Martin, C.A.; Burgess, D.S. Influence of β-lactam infusion strategy on acute kidney injury. Antimicrob. Agents Chemother. 2017, 61, e00871–17. [Google Scholar] [CrossRef] [PubMed]
- Costenaro, P.; Minotti, C.; Cuppini, E.; Barbieri, E.; Giaquinto, C.; Donà, D. Optimizing antibiotic treatment strategies for neonates and children: Does implementing extended or prolonged infusion provide any advantage? Antibiotics 2020, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Debray, A.; Callot, D.; Hirt, D.; et al. Beta-lactam exposure and safety in intermittent or continuous infusion in critically ill children: An observational monocenter study. Eur. J. Pediatr. 2023, 182, 965–973. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; et al. A multicenter randomized trial of continuous versus intermittent βlactam infusion in severe sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef]
- Imburgia, T.A.; Kussin, M.L. A Review of extended and continuous infusion beta-lactams in pediatric patients. J. Pediatr. Pharmacol. Ther. 2022, 27, 214–227. [Google Scholar] [CrossRef]
- Fan, S.Y.; Shum, H.P.; Cheng, W.Y.; Chan, Y.H.; Leung, S.M.; Yan, W.W. Clinical outcomes of extended versus intermittent infusion of piperacillin/tazobactam in critically ill patients: A prospective clinical trial. Pharmacotherapy 2017, 37, 109–119. [Google Scholar] [CrossRef]
- Rafati, M.R.; Rouini, M.R.; Mojtahedzadeh, M.; et al. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int. J. Antimicrob. Agents. 2006, 28, 122–127. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, J.; Oliveira, B.S.; Janeiro, S.; et al. Continuous infusion of piperacillin/tazobactam in septic critically ill patients--a multicenter propensity matched analysis. PLoS ONE 2012, 7, e49845. [Google Scholar] [CrossRef]
- Hyun, D.G.; Seo, J.; Lee, S.Y.; et al. Continuous piperacillintazobactam infusion improves clinical outcomes in critically ill patients with sepsis: A retrospective, singlecentre study. Antibiotics 2022, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Cutro, S.R.; Holzman, R.; Dubrovskaya, Y.; et al. Extendedinfusion versus standard-infusion piperacillin-tazobactam for sepsis syndromes at a tertiary medical center. Antimicrob. Agents Chemother. 2014, 58, 4470–4475. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cui, X.; Ma, Z.; Liu, L. Evaluation outcomes associated with alternative dosing strategies for piperacillin/tazobactam: A systematic review and metaanalysis. J. Pharm. Pharm. Sci. 2016, 19, 274–289. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.B.; et al. Betalactam infusion in severe sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Jaruratanasirikul, S.; Limapichat, T.; Jullangkoon, M.; Aeinlang, N.; Ingviya, N.; Wongpoowarak, W. Pharmacodynamics of meropenem in critically ill patients with febrile neutropenia and bacteraemia. Int. J. Antimicrob. Agents. 2011, 38, 231–236. [Google Scholar] [CrossRef]
- Ram, R.; Halavy, Y.; Amit, O.; et al. Extended vs. bolus infusion of broad-spectrum β- lactams for febrile neutropenia: An unblinded, randomized trial. Clin. Infect. Dis. 2018, 67, 1153–1160. [Google Scholar] [CrossRef]
- Knoderer, C.A.; Karmire, L.C.; Andricopulos, K.L.; Nichols, K.R. Extended infusion of piperacillin/tazobactam in children. J. Pediatr. Pharmacol. Ther. 2017, 22, 212–217. [Google Scholar] [CrossRef]
- Kebudi, R.; Kizilocak, H. Febrile neutropenia in children with cancer: Approach to diagnosis and treatment. Curr. Pediatr. Rev. 2018, 14, 204–209. [Google Scholar] [CrossRef]
- Solórzano-Santos, F.; Quezada-Herrera, A.; FuentesPacheco, Y.; et al. Piperacillin/tazobactam in continuous infusion versus intermittent infusion in children with febrile neutropenia. Rev. Invest. Clin. 2019, 71, 283–290. [Google Scholar] [CrossRef]
- Sprauten, P.F.; Beringer, P.M.; Louie, S.G.; Synold, T.W.; Gill, M.A. Stability and antibacterial activity of cefepime during continuous infusion. Antimicrob. Agents Chemother. 2003, 47, 1991–1994. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Limapichat, T.; Jullangkoon, M.; Aeinlang, N.; Ingviya, N.; Wongpoowarak, W. Pharmacodynamics of meropenem in critically ill patients with febrile neutropenia and bacteraemia. Int. J. Antimicrob. Agents. 2011, 38, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.N.; Mynatt, R.P.; Kaye, K.S.; Zhao, J.J.; Pogue, J.M. Clinical outcomes with extended versus intermittent infusion of anti-pseudomonal beta-lactams in patients with Gram-negative bacteremia. Open Forum Infect Dis. 2023, 10, ofad170. [Google Scholar] [CrossRef] [PubMed]
- Imburgia, T.A.; Kussin, M.L. A Review of extended and continuous infusion beta-lactams in pediatric patients. J. Pediatr. Pharmacol. Ther. 2022, 27, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A.; West, J.E.; O’Brien, J.M.; Goff, D.A. Extendedinfusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob. Agents Chemother. 2013, 57, 2907–2912. [Google Scholar] [CrossRef]
- Wrenn, R.H.; Cluck, D.; Kennedy, L.; Ohl, C.; Williamson, J.C. Extended infusion compared to standard infusion cefepime as empiric treatment of febrile neutropenia. J. Oncol. Pharm. Pract. 2018, 24, 170–175. [Google Scholar] [CrossRef]
- Courter, J.D.; Kuti, J.L.; Girotto, J.E.; Nicolau, D.P. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr. Blood Cancer. 2009, 53, 379–385. [Google Scholar] [CrossRef]
- Shoji, K.; Bradley, J.S.; Reed, M.D.; van den Anker, J.N.; Domonoske, C.; Capparelli, E.V. Population pharmacokinetic assessment and pharmacodynamic implications of pediatric cefepime dosing for susceptibledose-dependent organisms. Antimicrob. Agents Chemother. 2016, 60, 2150–2156. [Google Scholar] [CrossRef]
- Nichols, K.R.; Karmire, L.C.; Cox, E.G.; Kays, M.B.; Knoderer, C.A. Implementing extended-infusion cefepime as standard of care in a children’s hospital: A prospective descriptive study. Ann. Pharmacother. 2015, 49, 419–426. [Google Scholar] [CrossRef]
- Zembles, T.N.; Schortemeyer, R.; Kuhn, E.M.; Bushee, G.; Thompson, N.E.; Mitchell, M.L. Extended infusion of betalactams is associated with improved outcomes in pediatric patients. J. Pediatr. Pharmacol. Ther. 2021, 26, 187–193. [Google Scholar] [CrossRef]
- Chytra, I.; Stepan, M.; Benes, J.; et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: A randomized open-label controlled trial. Crit Care. 2012, 6, R113. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Gu, J.; Lyu, J.; et al. Pharmacokinetic and pharmacodynamic efficacies of continuous versus intermittent administration of meropenem in patients with severe sepsis and septic shock: A prospective randomized pilot study. Chin. Med. J. 2017, 130, 1139–1145. [Google Scholar] [CrossRef]
- Monti, G.; Bradic, N.; Marzaroli, M.; et al. Continuous vs. intermittent meropenem administration in critically ill patients with sepsis: The MERCY randomized clinical trial. JAMA 2023, 330, 141–151. [Google Scholar] [CrossRef]
- Dräger, S.; von Rotz, M.; Labhardt, N.D.; et al. Early target attainment with continuous infusion meropenem and piperacillin/tazobactam and utilization of therapeutic drug monitoring in critically ill patients: A retrospective cohort study from 2017 to 2020. Open Forum Infect Dis. 2023, 10, ofad143. [Google Scholar] [CrossRef]
- Tournayre, S.; Mathieu, O.; Villiet, M.; et al. Factors associated with meropenem pharmacokinetic/pharmacodynamic target attainment in septic critically ill patients treated with extended intermittent infusion or continuous infusion. Int. J. Antimicrob. Agents. 2023, 62, 106868. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Cojutti, P.; Pea, F. Pharmacokinetics and pharmacodynamics of continuous infusion meropenem in overweight, obese, and morbidly obese patients with stable and unstable kidney function: A step toward dose optimization for the treatment of severe gram-negative bacterial infections. Clin. Pharmacokinet. 2015, 54, 93341. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Lipman, J.; Akova, M.; et al. Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J. Antimicrob. Chemother. 2016, 71, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Jen, S.P.; Altshuler, D.; Papadopoulos, J.; Pham, V.P.; Dubrovskaya, Y. Evaluation of meropenem extended versus intermittent infusion dosing protocol in critically ill patients. J. Intensive Care Med. 2020, 35, 763–771. [Google Scholar] [CrossRef]
- Dräger, S.; von Rotz, M.; Labhardt, N.D.; et al. Early target attainment with continuous infusion meropenem and piperacillin/tazobactam and utilization of therapeutic drug monitoring in critically ill patients: A retrospective cohort study from 2017 to 2020. Open Forum Infect Dis. 2023, 10, ofad143. [Google Scholar] [CrossRef]
- Wong, G.; Taccone, F.; Villois, P.; et al. β-lactam pharmacodynamics in Gram-negative bloodstream infections in the critically ill. J. Antimicrob. Chemother. 2020, 75, 429–433. [Google Scholar] [CrossRef]
- Tournayre, S.; Mathieu, O.; Villiet, M.; et al. Factors associated with meropenem pharmacokinetic/pharmacodynamic target attainment in septic critically ill patients treated with extended intermittent infusion or continuous infusion. Int. J. Antimicrob. Agents. 2023, 62, 106868. [Google Scholar] [CrossRef]
- Yu, Z.; Pang, X.; Wu, X.; Shan, C.; Jiang, S. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: A meta-analysis. PLoS ONE 2018, 13, e0201667. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Clinical outcomes of continuous vs. intermittent meropenem infusion for the treatment of sepsis: A systematic review and metaanalysis. Adv. Clin. Exp. Med. 2020, 29, 993–1000. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Lazzarotto, D.; Candoni, A.; et al. Real-time TDM-based optimization of continuous-infusion meropenem for improving treatment outcome of febrile neutropenia in oncohaematological patients: Results from a prospective, monocentric, interventional study. J. Antimicrob. Chemother. 2020, 75, 3029–3037. [Google Scholar] [CrossRef]
- Fehér, C.; Rovira, M.; Soriano, A.; et al. Effect of meropenem administration in extended infusion on the clinical outcome of febrile neutropenia: A retrospective observational study. J. Antimicrob. Chemother. 2014, 69, 2556–2562. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, J.; Oliveira, B.S.; Janeiro, S.; et al. Continuous infusion of piperacillin/tazobactam in septic critically ill patients--a multicenter propensity matched analysis. PLoS ONE 2012, 7, e49845. [Google Scholar] [CrossRef]
- Debray, A.; Callot, D.; Hirt, D.; et al. Beta-lactam exposure and safety in intermittent or continuous infusion in critically ill children: An observational monocenter study. Eur. J. Pediatr. 2023, 182, 965–973. [Google Scholar] [CrossRef]
- Zembles, T.N.; Kuhn, E.M.; Thompson, N.E.; Mitchell, M.L. Extended infusion β-lactams for the treatment of gramnegative bacteremia in children. J. Pediatr. Pharmacol. Ther. 2022, 27, 677–681. [Google Scholar] [CrossRef]
- Cao, G.; Zhou, P.; Zhang, H.; Sun, B.; Tong, X.; Xing, Y. Extended infusion of meropenem in neonatal sepsis: A historical cohort study. Antibiotics 2022, 11, 341. [Google Scholar] [CrossRef]
- Leegwater, E.; Wewerinke, L.; de Grauw, A.M.; van Veen, M.; Storm, B.N.; Kruizinga, M.D. Optimization of β-lactam dosing regimens in neonatal infections: Continuous and extended administration versus intermittent administration. Clin. Pharmacokinet. 2023, 62, 715–724. [Google Scholar] [CrossRef]
- Hong, L.T.; Downes, K.J.; FakhriRavari, A.; et al. International consensus recommendations for the use of prolonged-infusion beta-lactam antibiotics: Endorsed by the American College of Clinical Pharmacy, British Society for Antimicrobial Chemotherapy, Cystic Fibrosis Foundation, European Society of Clinical Microbiology and Infectious Diseases, Infectious Diseases Society of America, Society of Critical Care Medicine, and Society of Infectious Diseases Pharmacists. Pharmacotherapy 2023, 43, 740–777. [Google Scholar] [CrossRef]
- Chen, M.; Buurma, V.; Shah, M.; Fahim, G. Evaluation of studies on extended versus standard infusion of betalactam antibiotics. Am. J. Health Syst. Pharm. 2019, 76, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Grupper, M.; Kuti, J.L.; Nicolau, D.P. Continuous and prolonged intravenous β-lactam dosing: Implications for the clinical laboratory. Clin. Microbiol. Rev. 2016, 29, 75972. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Dulhunty, J.M.; Bellomo, R.; Lipman, J.; Roberts, J.A. Continuous beta-lactam infusion in critically ill patients: The clinical evidence. Ann. Intensive Care. 2012, 2, 37. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
© GERMS 2025.
Share and Cite
Alawyia, B.; Fathima, S.; Spernovasilis, N.; Alon-Ellenbogen, D. Continuous Versus Intermittent Infusion of Beta-Lactam Antibiotics: Where Do We Stand Today? A Narrative Review. Germs 2024, 14, 162-178. https://doi.org/10.18683/germs.2024.1428
Alawyia B, Fathima S, Spernovasilis N, Alon-Ellenbogen D. Continuous Versus Intermittent Infusion of Beta-Lactam Antibiotics: Where Do We Stand Today? A Narrative Review. Germs. 2024; 14(2):162-178. https://doi.org/10.18683/germs.2024.1428
Chicago/Turabian StyleAlawyia, Basil, Sarah Fathima, Nikolaos Spernovasilis, and Danny Alon-Ellenbogen. 2024. "Continuous Versus Intermittent Infusion of Beta-Lactam Antibiotics: Where Do We Stand Today? A Narrative Review" Germs 14, no. 2: 162-178. https://doi.org/10.18683/germs.2024.1428
APA StyleAlawyia, B., Fathima, S., Spernovasilis, N., & Alon-Ellenbogen, D. (2024). Continuous Versus Intermittent Infusion of Beta-Lactam Antibiotics: Where Do We Stand Today? A Narrative Review. Germs, 14(2), 162-178. https://doi.org/10.18683/germs.2024.1428




