Clinical, Epidemiological and Molecular Aspects of Patients with Mpox in Romania
Abstract
Introduction
Methods
Participants and Samples
Real Time PCR
Whole-Genome Sequencing (WGS)
Phylogenetic Analysis
Screening for APOBEC Induced Mutations
Results
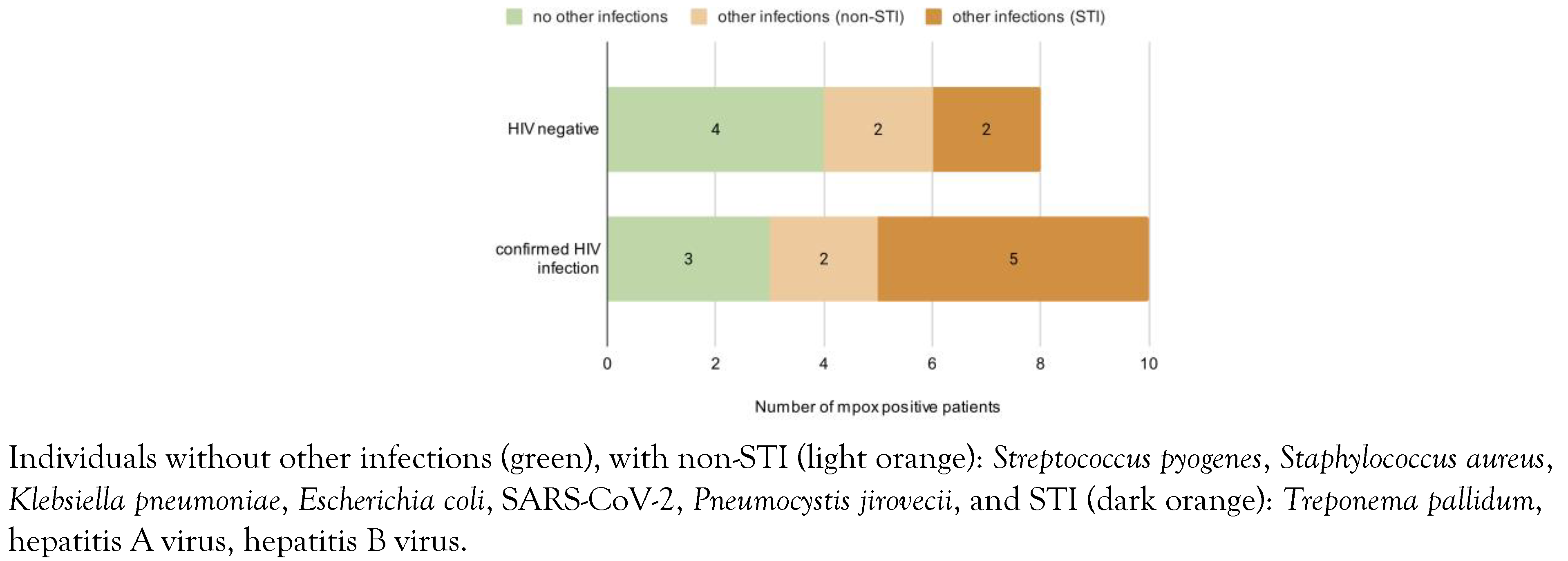
Clinical and Epidemiological Characteristics of Patients Positive for Mpox
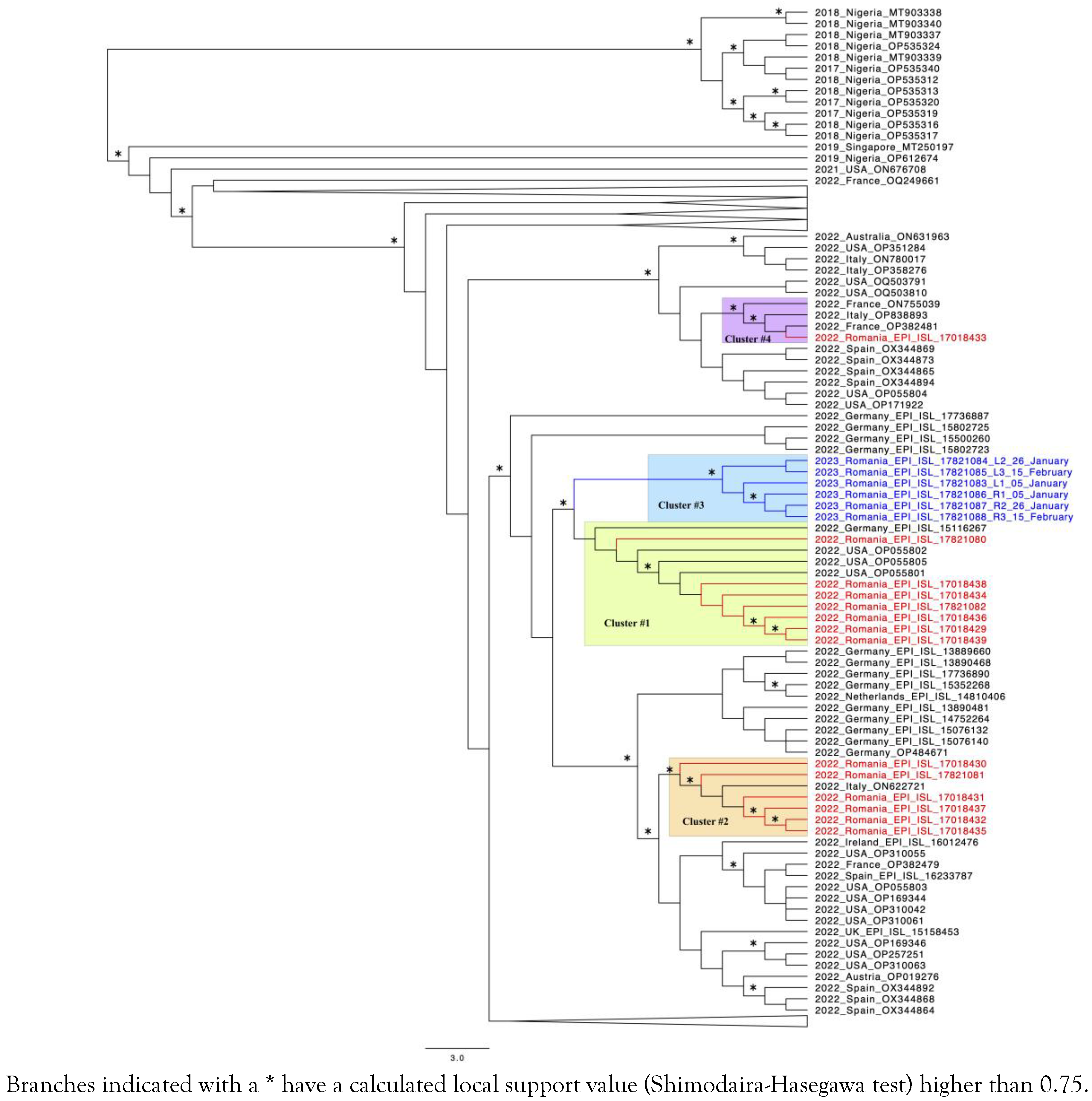
Phylogenetic Analysis
Mutational Screening
Discussion
Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval and Consent
Nucleotide accession numbers
References
- Breman, J.G.; Kalisa-Ruti Steniowski, M.V.; Zanotto, E.; Gromyko, A.I.; Arita, I. Human monkeypox, 1970-79. Bull World Health Organ. 1980, 58, 165–182. [Google Scholar] [PubMed]
- Reynolds, M.G.; Davidson, W.B.; Curns, A.T.; et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg Infect Dis. 2007, 13, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Nigeria Centre for Disease Control. Update on Monkeypox (MPX) in Nigeria. NCDC, 19 Jun 2022. 2022. Available online: https://ncdc.gov.ng/themes/common/files/sitreps/4f9bc59b967d1f4b19d61358595d3546.pdf (accessed on 24 April 2024).
- World Health Organization. Mpox (monkeypox). 2023. Available online: https://www.who.int/news-room/factsheets/detail/monkeypox (accessed on 29 May 2024).
- Jezek, Z.; Szczeniowski, M.; Paluku, K.M.; Mutombo, M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bunge, E.M.; Hoet, B.; Chen, L.; et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Bhattacharya, M.; Sharma, A.R.; Dhama, K. Evolution, epidemiology, geographical distribution, and mutational landscape of newly emerging monkeypox virus. Geroscience 2022, 44, 2895–2911. [Google Scholar] [CrossRef] [PubMed]
- Isidro, J.; Borges, V.; Pinto, M.; et al. Addendum: Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Oprea, C.; Ianache, I.; Piscu, S.; et al. First report of monkeypox in a patient living with HIV from Romania. Travel Med Infect Dis. 2022, 49, 102395. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 2022 Global Map & Case Count. 2024. Available online: https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html (accessed on 29 May 2024).
- Seemann, T. Shovill. Available online: https://github.com/tseemann/shovill (accessed on 4 August 2020).
- Rose, P.P.; Korber, B.T. Detecting hypermutations in viral sequences with an emphasis on G --> A hypermutation. Bioinformatics. 2000, 16, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, E.; Herrera, C.; Sabino-Santos, G. Monkeypox virus evolution before 2022 outbreak. Emerg Infect Dis. 2023, 29, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Adetifa, I.; Muyembe, J.J.; Bausch, D.G.; Heymann, D.L. Mpox neglect and the smallpox niche: a problem for Africa, a problem for the world. Lancet 2023, 401, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fu, L.; Wang, B.; et al. Clinical characteristics of human mpox (monkeypox) in 2022: a systematic review and meta-analysis. Pathogens 2023, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.; LePage, T.; Bester, V.; Yoon, H.; Browne, F.; Nemec, E.C. Mpox (formally known as monkeypox). Physician Assist Clin. 2023, 8, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Candela, C.; Raccagni, A.R.; Bruzzesi, E.; et al. Human monkeypox experience in a tertiary level hospital in Milan, Italy, between May and October 2022: epidemiological features and clinical characteristics. Viruses 2023, 15, 667. [Google Scholar] [CrossRef] [PubMed]
- Okoli, G.N.; Van Caeseele, P.; Askin, N.; Abou-Setta, A.M. Comparative evaluation of the clinical presentation and epidemiology of the 2022 and previous Mpox outbreaks: a rapid review and meta-analysis. Infect Dis (Lond). 2023, 55, 490–508. [Google Scholar] [CrossRef] [PubMed]
- European Union Agency for Fundamental Rights. EU LGBTI survey II - A long way to go for LGBTI equality. Available online: https://fra.europa.eu/sites/default/files/fra_uploads/lgbti-survey-country-data_romania.pdf (accessed on 14 May 2020).
- Compartment for monitoring and evaluating HIV/AIDS infection in Romania (CNLAS). “Evolution of HIV in Romania”. Available online: https://www.cnlas.ro/images/doc/31122022_rom.pdf (accessed on 31 December 2022).
- European Centre for Disease Control. Factsheet for health professionals on Mpox (monkeypox). Available online: https://www.ecdc.europa.eu/en/all-topicsz/monkeypox/factsheet-health-professionals (accessed on 25 October 2022).
- Pecori, R.; Di Giorgio, S.; Paulo Lorenzo, J.; Nina Papavasiliou, F. Functions and consequences of AID/APOBEC-mediated DNA and RNA deamination. Nat Rev Genet. 2022, 23, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Annan Sudarsan, A.K.; Gaba, A.; Chelico, L. Stability of APOBEC3F in the presence of the APOBEC3 antagonist HIV-1 Vif increases at the expense of co-expressed APOBEC3H haplotype I. Viruses 2023, 15, 463. [Google Scholar] [CrossRef] [PubMed]
- Suspène, R.; Raymond, K.A.; Boutin, L.; et al. APOBEC3F is a mutational driver of the human monkeypox virus identified in the 2022 outbreak. J Infect Dis. 2023, 228, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Refsland, E.W.; Stenglein, M.D.; Shindo, K.; Albin, J.S.; Brown, W.L.; Harris, R.S. Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues: implications for HIV-1 restriction. Nucleic Acids Res. 2010, 38, 4274–4284. [Google Scholar] [CrossRef] [PubMed]
| Clinical Signs and Symptoms | N (%) |
|---|---|
| Fever | 11 (61%) |
| Rash | 10 (55%) |
| Lymphadenopathy | 10 (55%) |
| Oral lesions | 9 (50%) |
| Genital lesions | 8 (44%) |
| Fatigue | 7 (38%) |
| Chills / sweating | 7 (38%) |
| Myalgia | 6 (33%) |
| Anal lesions | 4 (22%) |
| Dysphagia | 4 (22%) |
| Hospitalization | 2 (11%) |
| Headache | 1 (6%) |
| Cough / other respiratory symptoms | 1 (6%) |
© GERMS 2024.
Share and Cite
Hohan, R.; Vlaicu, O.; Bănică, L.; Tudor, A.I.; Negru, A.; Paraschiv, S.; Oţelea, D. Clinical, Epidemiological and Molecular Aspects of Patients with Mpox in Romania. Germs 2024, 14, 126-135. https://doi.org/10.18683/germs.2024.1425
Hohan R, Vlaicu O, Bănică L, Tudor AI, Negru A, Paraschiv S, Oţelea D. Clinical, Epidemiological and Molecular Aspects of Patients with Mpox in Romania. Germs. 2024; 14(2):126-135. https://doi.org/10.18683/germs.2024.1425
Chicago/Turabian StyleHohan, Robert, Ovidiu Vlaicu, Leontina Bănică, Andreea Ioana Tudor, Anca Negru, Simona Paraschiv, and Dan Oţelea. 2024. "Clinical, Epidemiological and Molecular Aspects of Patients with Mpox in Romania" Germs 14, no. 2: 126-135. https://doi.org/10.18683/germs.2024.1425
APA StyleHohan, R., Vlaicu, O., Bănică, L., Tudor, A. I., Negru, A., Paraschiv, S., & Oţelea, D. (2024). Clinical, Epidemiological and Molecular Aspects of Patients with Mpox in Romania. Germs, 14(2), 126-135. https://doi.org/10.18683/germs.2024.1425




