Introduction
Multidrug resistant (MDR) Gram-negative infections are a major cause of concern in India. This concern is further worsened in neutropenic patients as they require prompt initiation of empiric antibiotics to cover these MDR 1infections. Hematology-oncology patients (acute myeloid leukemia – AML, acute lymphoblastic leukemia – ALL, allogenic stem cell transplant recipients) have prolonged neutropenia and may have multiple episodes of infections; as a consequence, these patients are exposed to antibiotics, making them colonized with MDR organisms. Carbapenem-resistant Enterobacteriaceae (CRE) constitute majority of the MDR infections in hematology-oncology settings [
1,
2]. Therapy for CRE includes toxic and expensive antibiotics such as colistin, tigecycline, parenteral fosfomycin, ceftazidime-avibactam. Not only is the therapy for CRE expensive and toxic, but CRE infections are per se associated with poor outcomes with mortality rates ranging from 20% to 50% [
3]. Many publications have been done, which have shown that antimicrobial stewardship (AMS) implementation in hematology-oncology settings helps in reducing antibiotic usage and incidence of MDR bugs without adverse effects on outcomes [
4,
5]. As clinicians, we need to find a balance between good early empirical antibiotic therapy in febrile neutropenia and overuse of antibiotics. ECIL-4 (European Council on Infections in Leukemia) guidelines, therefore recommend de-escalation of antibiotics in a stable febrile neutropenic patient in whom cultures are negative and also early discontinuation of antibiotics if the patient is afebrile irrespective of neutrophil recovery [
6].
The burden of MDR and in particular CRE is huge in India, which in a way encourages clinicians to step up to CRE antibiotics in patients with neutropenia. Since gut translocation is the predominant factor responsible for bloodstream infection in neutropenic hosts, patients exposed to broad spectrum antibiotics subsequently get infections with MDR organisms. With this in the background, we started implementing ECIL-4 recommendations in our hematology-oncology unit. We encouraged not escalating antibiotics to CRE drugs (colistin, fosfomycin, tigecycline, minocycline, ceftazidime-avibactam) for only fever and stopping these drugs if the patient remained stable and the cultures were negative. As with any AMS implementation strategy, execution is not perfect and for some of the patients, suggestions given by AMS team were not followed by the primary (admitting physicians) team, which include avoiding use of empirical CRE antibiotics for just fever or de-escalation of antibiotics if cultures are negative or does not show CRE. We therefore planned a retrospective study to analyze the outcomes of CRE bloodstream infections and to analyze the predictors of mortality in patients with CRE bacteremia.
Methods
This was a retrospective study conducted in a hematology unit of a dedicated tertiary level oncology center in North India. The study was conducted from April 2019 to December 2021.
Patients
Our inclusion criteria were: presence of carbapenem-resistant Enterobacteriaceae bacteremia (blood culture positive) and neutropenia. We excluded patients who had febrile neutropenia without blood culture positivity, blood culture positive for organisms other than CRE or blood culture positive for CRE but not fitting into febrile neutropenia and if follow-up was less than 72 hours. Patients whose blood culture showed poly-microbial growth were also excluded from the study. We collected data including age, sex, type of malignancy, transplant status, sequential (sepsis-related) organ failure assessment (SOFA) score at onset of bacteremia, peak SOFA score, antibiotic prescriptions for last 14 days and then targeted therapy, duration of neutropenia, outcome at 28 days.
Definitions
We divided patients into antibiotic stewardship cohort and non-stewardship cohort. The antibiotic stewardship cohort was comprised of those patients who were not exposed to CRE drugs or if started, CRE drugs (colistin, fosfomycin, tigecycline, minocycline, ceftazidime-avibactam; either alone or in combination) were stopped (within 72 hours) if cultures were negative as per suggestions of the AMS team. The non-stewardship cohort was comprised of patients who were continued on CRE drugs for more than 72 hours. Other antibiotic stewardship (AMS) interventions included suggestions by AMS team on targeted therapy, correct dose and duration but these variables were not analysed in this study. Further, not all suggestions (particularly advice to de-escalation of antibiotics) were followed by the primary team, hence the patients in whom de-escalation advice was followed were included in the AMS cohort and those in whom de-escalation advice was not followed were included in the non-stewardship cohort.
Statistical analysis
Intergroup analysis was done using student the t-test (for continuous variables) and chi-square test (for discrete variables) for comparisons between the antibiotic stewardship cohort and the non-stewardship cohort. Binary logistic regression analysis was done taking outcome at 28 days as dependent variable to study the role of independent variables in predicting mortality using SPSS software version 22 (IBM Corp., USA).
Results
A total of total 935 febrile neutropenia episodes in 656 patients were observed during the study period, out of which only 278 episodes had positive blood culture. Among these 278 bacteremias, 90 were non-CRE bacteremia, 29 were non-Enterobacteriaceae and 20 were Gram positive cocci (
Staphylococcus aureus or enterococci). Only 139 patients had mono-microbial bloodstream infections due to CRE, which were included in the analysis (
Table 1).
The median age of the study population (n=139) was 41 years (range 13-74 years), of which 82 (58.9%) were males. The most common malignant condition was acute myeloid leukemia (92 patients) followed by myeloma (12 patients) and lymphoma (12 patients). Forty-six patients (33.1%) were post stem cell transplant and 41 (97.8%) of these were post allogenic transplant. The median time span since transplant was 25 days (range 8-324 days). The median SOFA score at the onset of bacteremia was 6 (range 4-10) and the median peak SOFA score during the course of therapy was 8 (range 4-14). The median duration of neutropenia was 20 days (range 4-100 days).
Klebsiella pneumoniae was the most common organism, seen in 109 patients (78.4%), followed by E. coli, seen in 28 patients (20.1%) and 2 patients had Enterobacter cloacae. Only 69 isolates were tested for carbapenemase gene by Xpert CARBA-R, OXA-48 was detected in 24 isolates (34.7%). Combination of New Delhi metallo-β-lactamase (NDM) and OXA-48 was detected in 32 isolates (46.3%). Only NDM was seen in 13 (18.8%) of isolates.
With regard to targeted therapy for CRE bacteremia, colistin based combination therapy was used in 80 (57.5%) patients, ceftazidime-avibactam alone in 9 (6.4%) and ceftazidime-avibactam in combination with aztreonam in 49 (35.2%). Among patients with colistin based combination therapy, the most commonly used combination was colistin + IV fosfomycin (in 36 patients) followed by colistin + tigecycline (in 26 patients).
On studying antibiotic prescriptions, we found that all patients had been exposed to antibiotics in past 14 days prior to the onset of bacteremia. Cefoperazone-sulbactam alone was used in 35 (25.1%) patients, cefoperazone-sulbactam plus amikacin in 9 (6.4%), meropenem alone in 25 (17.9%), meropenem plus amikacin in 12 (8.6%). A total of 60 (43.1%) patients were initiated on CRE therapy, out of which for 18 patients CRE drugs were stopped within 72 hours. On studying the reason for usage of antibiotics in these patients, we found that 10 (7.6%) patients had microbiologically documented infection (MDI), 19 (13.6%) patients had clinically documented infection (CDI) and for 110 (79.1%) patients antibiotics were given for only fever.
All-cause mortality at 28 days was observed in 37 patients (26.6%) and for one patient the outcome could not be recorded as he was lost to follow-up.
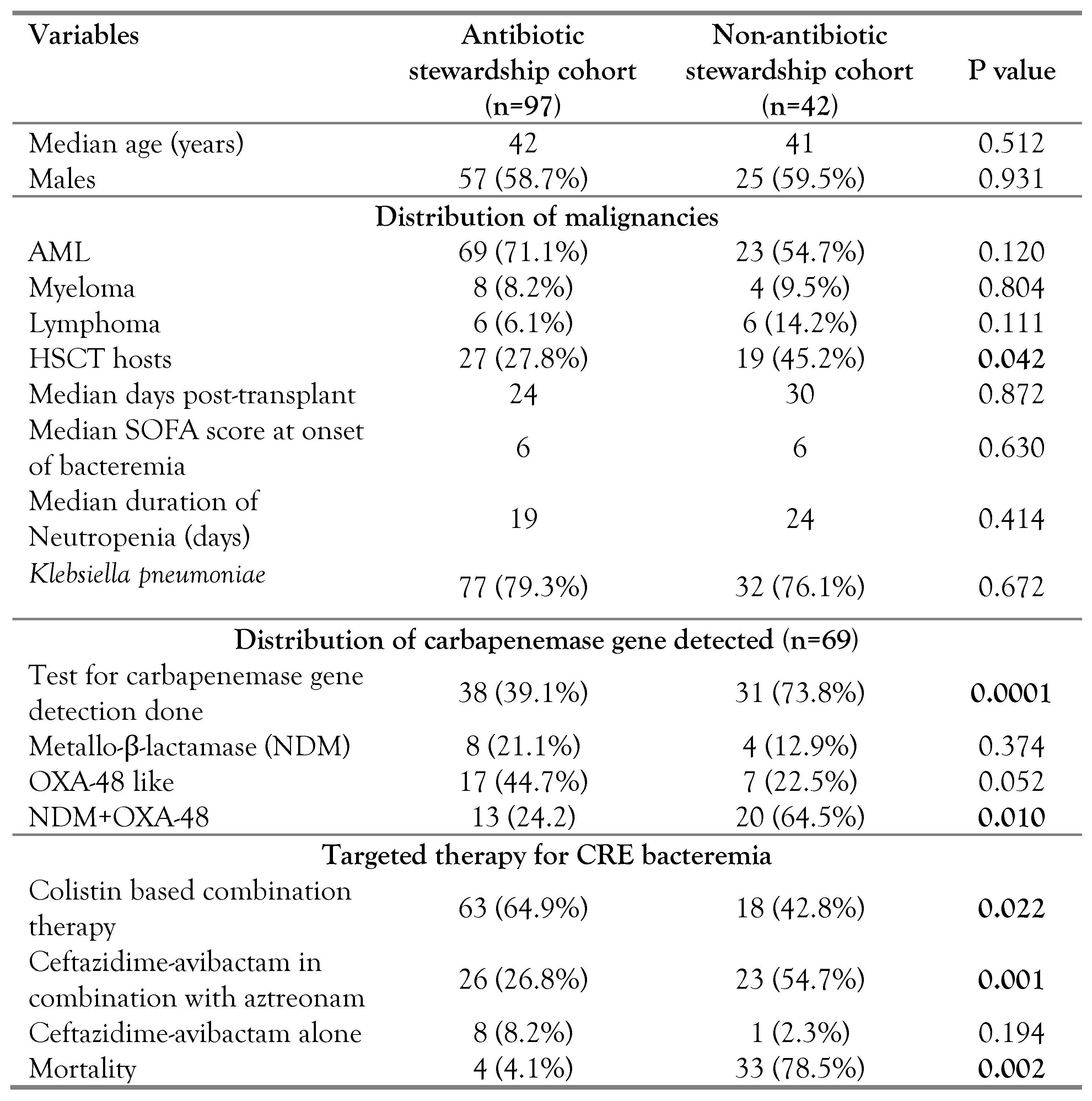
Ninety-seven patients were included in the antibiotic stewardship cohort and 42 patients were included in the non-stewardship cohort. On intergroup analysis between the antibiotic stewardship cohort and the non-antibiotic stewardship cohort (
Table 2), we found that the number of post stem cell transplant hosts were significantly higher in the non-antibiotic stewardship cohort (27.8% vs 45.2%; p<0.05). Mortality was significantly higher in the non-antibiotic stewardship cohort (4.1% vs 78.5%; p<0.05). the rest of the variables, such as age, sex, type of malignancy, duration of neutropenia, and SOFA score at onset and peak were comparable across the two cohorts.
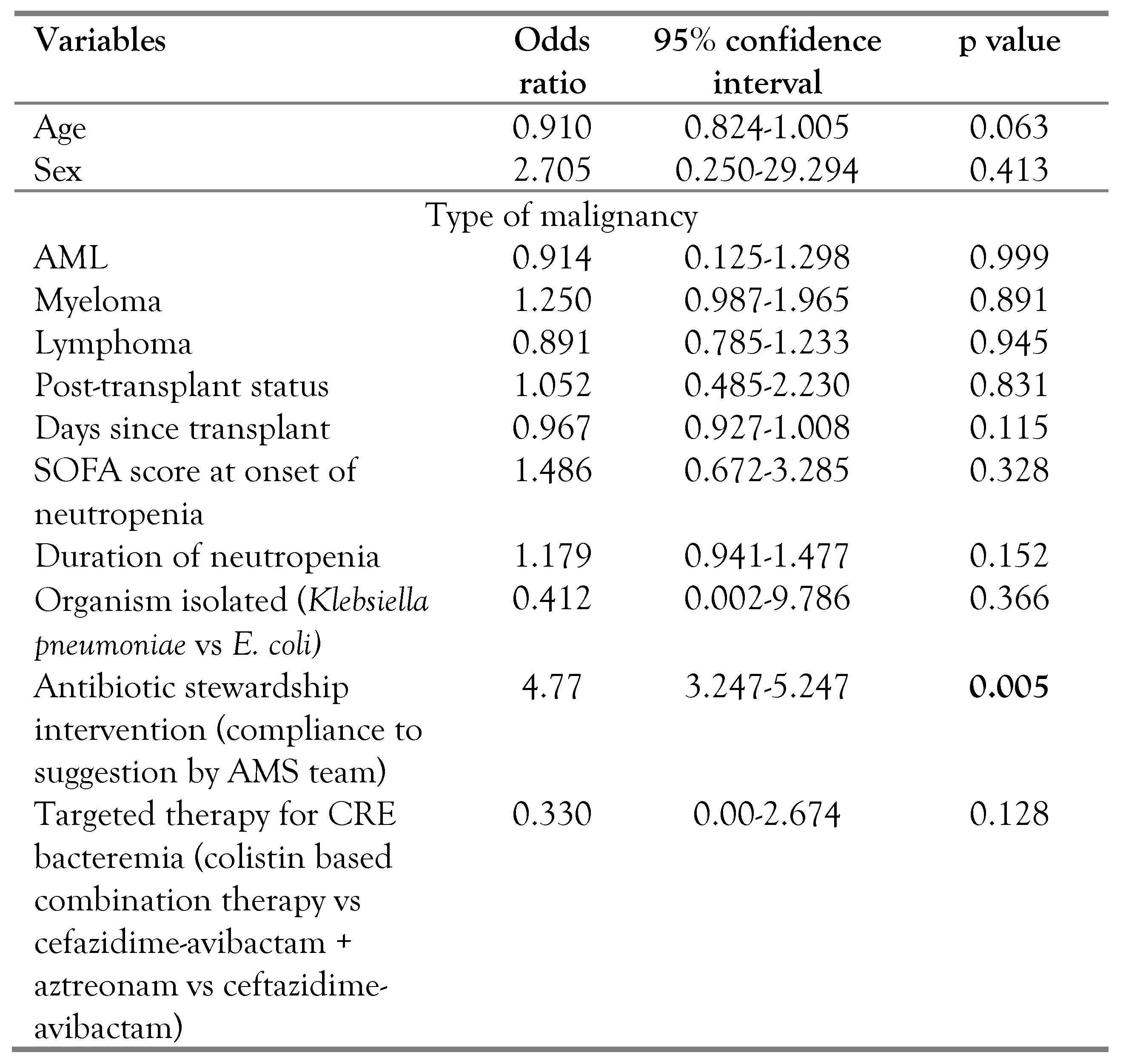
On binary logistic regression analysis (
Table 3), lack of antibiotic stewardship intervention was an independent predictor of mortality.
Discussion
The Infectious Disease Society of America (IDSA) [
7] has recently updated their guidelines for management of CRE and have recommended usage of ceftazidime avibactam alone for KPC and OXA-48-like, whereas recommendations for metallo-β-lactamase include combination of ceftazidime avibactam with aztreonam. Cefiderocol or plazomycin are other alternatives recommended, both of which are not available in India. Colistin is not the preferred choice any more for management of CRE. These guidelines were published in 2020 and our practice also shifted from colistin based combination therapy to use of ceftazidime avibactam with aztreonam over the study period [
8]. Data on use of combination of ceftazidime-avibactam and aztreonam in hematology-oncology patients for NDM or NDM+OXA-48 is very scarce and our study provides some evidence for the same.
Many studies have reported CRE bloodstream infections in hematology-oncology settings and have found very high mortality rate, we also found that close to 30% of our patients died at day 28. In this study, prior exposure to CRE drugs (lack of compliance with suggestion by AMS team for de-escalation) predicted mortality, which is line with the available literature [
9,
10]. The number of stem cell transplant recipients were higher in the non-stewardship cohort, this could be because compliance with suggestion to de-escalate was not considered by transplant physicians in this high risk group as de-escalation of antibiotics is not a very common practice in the Indian setting [
11].
With ever increasing prevalence of highly drug resistant pathogens, intervention to cut down on the usage of antibiotics is the need of the hour regardless of the patient profile. AMS has been the prime method to cut down the usage, but common AMS interventions like reducing duration of antibiotics for treatment of proven infection, stopping antibiotic coverage if a non-infectious etiology or a viral pathogen is identified is not easily applicable to the neutropenic setting due to lack of good literature favoring the same. ECIL-4 recommendations and other studies [
4,
5] do give us encouragement to practice the same, but it needs to be kept in mind that these studies are done in settings with low prevalence of CRE and they use antibiotics as prophylaxis during neutropenia, which is not a practice at our institute. In India, rates of CRE in tertiary care centers can range from 20-50% [
12]. As per our knowledge and literature search, this is the first study from a setting with high prevalence of CRE which demonstrates that AMS interventions can reduce mortality.
Our study has many limitations. First, it is a single center retrospective study, which may lead to inclusion bias and non-applicability to other centers. Second, we analyzed only all-cause mortality; death could have occurred because of other reasons as well, and it may have led to under- or over-reporting of mortality benefit of antibiotic stewardship. Lastly, no antibiotic stewardship study is complete without information on incidence of MDR organisms across the study period and overall antibiotic consumption data, which we fail to report. Despite, its limitations, this study gives practical real-world information on management of hematology-oncology patients infected with CRE.
Conclusions
This is the first study giving evidence that antibiotic stewardship is practically possible in high CRE setting and also that antibiotic stewardship can actually improve outcomes. This study will give clinicians or oncologists practicing in settings like ours some confidence in reducing empirical usage of CRE drugs.
Author Contributions
NB collected the data and wrote the manuscript. DB, NA, RA, NS, PM and RH critically analyzed the data and helped in finalizing the manuscript. All authors reviewed the manuscript. All authors read and approved the final version of the manuscript.
Institutional Review Board Statement
The study was approved by the Institute Ethics Committee (IEC) and patient consent was waived as it is a retrospective study with no intervention (IRB no: RGCIRC/IRB-BHR/137/2021; Dated December 12, 2021).
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
We would like to acknowledge the inputs of Ms. Vandana and Ms. Kavita from Microbiology Laboratory and also Dr. Ram Gopalakrishnan and Dr. Kalpesh Sukhwani.
Conflicts of Interest
All authors – none to declare.
References
- Barman, P.; Choudhary, D.; Chopra, S.; Thukral, T. Blood stream infections in hematopoietic stem cell transplant patients: A 2-year study from India. Oncol J India 2020, 4, 43–48. [Google Scholar] [CrossRef]
- Kumar, A.; Mohapatra, S.; Bakhshi, S.; et al. Rectal carriage of carbapenem-resistant Enterobacteriaceae: A menace to highly vulnerable patients. J Glob Infect Dis 2018, 10, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J.; Cohen, N.; Ma, K.C.; et al. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J Infect 2016, 73, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.; Moshier, E.; Jacobs, S.E.; et al. Practicing antimicrobial stewardship: De-escalating antibiotics in patients with acute myeloid leukemia and neutropenic fever. Open Forum Infect Dis 2020, 7, ofaa138. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, A.; Jansens, H.; Goossens, H.; et al. Safety and efficacy of antibiotic de-escalation and discontinuation in high-risk haematological patients with febrile neutropenia: A single-centre experience. Open Forum Infect Dis 2021, 9, ofab624. [Google Scholar] [CrossRef] [PubMed]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2014, 99, 400. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E). Carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis 2021, 72, e169–e183. [Google Scholar] [CrossRef] [PubMed]
- Sahu, C.; Pal, S.; Patel, S.S.; Singh, S.; Gurjar, M.; Ghoshal, U. Phenotypic synergy testing of ceftazidime-avibactam with aztreonam in a university hospital having high number of metallobetalactamase producing bacteria. Infect Dis (Lond) 2020, 52, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Gudiol, C.; Tubau, F.; Calatayud, L.; et al. Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: Risk factors, antibiotic therapy and outcomes. J Antimicrob Chemother 2011, 66, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Demandt, A.M.P.; Geerse, D.A.; Janssen, B.J.P.; Winkens, B.; Schouten, H.C.; van Mook, W.N.K.A. The prognostic value of a trend in modified SOFA score for patients with hematological malignancies in the intensive care unit. Eur J Haematol 2017, 99, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Walia, K.; Ohri, V.C.; Mathai, D.; Antimicrobial Stewardship Programme of ICMR. Antimicrobial stewardship programme (AMSP) practices in India. Indian J Med Res 2015, 142, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Indian Council of Medical Research. Antimicrobial Resistance Research and Surveillance Network Annual report 2020. Available online: https://main.icmr.nic.in/sites/default/files/guidelines/ AMRSN_annual_report_2020.pdf (accessed on 21 January 2020).
Table 1.
Various parameters of the study population.
Table 1.
Various parameters of the study population.
Table 2.
Various parameters between the stewardship cohort and the non-antibiotic stewardship cohort.
Table 2.
Various parameters between the stewardship cohort and the non-antibiotic stewardship cohort.
Table 3.
Multivariate regression analysis between mortality and alive status at 28 days.
Table 3.
Multivariate regression analysis between mortality and alive status at 28 days.