Abstract
Introduction: Infectious vaginitis is prevalent in developing countries. Most of the females suffer from vaginal infections at least once per lifetime. Due to limited resources, many infections are misdiagnosed or undiagnosed. Good diagnosis of these infections is critically important and will definitely help to guide treatment and prevent recurrence. Methods: A total of 1080 vaginal swabs were collected from symptomatic females. Nugent’s score and Amsel’s criteria were applied to diagnose bacterial vaginosis (BV). A rapid test was used to identify Gardnerella vaginalis. Trichomonal vaginitis (TV) was diagnosed through microscopic examination. Vulvovaginal candidiasis (VVC) was also identified microscopically and using conventional culture. Finally, aerobic vaginitis (AV) was detected using Donder’s scale combined with conventional culture and biochemical tests. Results: There was no statistically significant association between age and type of vaginal infection (p = 0.130). Vulvovaginal inflammation, itching and redness were significantly associated with VVC (p ≤ 0.012). BV was detected as single infection in 43.8%, followed by VVC 24.2%. On the contrary, AV and TV were scarcely detected among the participants; 4.9% and 0.5% respectively. Mixed infections between BV and VVC were noted in 26.6%. Conclusions: BV showed the highest prevalence followed by VVC. Mixed infections between BV and VVC were evidently noted, therefore good reliable diagnosis using cost-effective methods is crucial for proper treatment. Aerobic vaginitis showed low prevalence and most of the Streptococcus spp. were isolated from pregnant females. The low prevalence of Trichomonas vaginalis may be due to the dependance on conventional methods for diagnosis, and thus more advanced diagnostic tools are required.
Introduction
Different infectious and non-infectious factors can cause vaginal inflammation, or vaginitis [1]. Vulvovaginal candidiasis (VVC), bacterial vaginosis (BV), and trichomonal vaginitis (TV) are the most prevalent causes of vaginal infections [2]. Lactobacilli are the dominant normal flora of the healthy vagina and their utmost function is to protect the vaginal tract from invading pathogenic bacteria. Consequently, any disturbance in the balance of the lactobacilli will lead to an elevation in anaerobes, predisposing to BV [3]. BV is the most common cause of homogeneous grayish-white thin smelly discharge. The prevalence of BV, depending on ethnicity, socio-economic status and geographical location, ranges from 8 to 51% [4]. VVC is the second most common vaginal infection affecting females of childbearing age. Approximately 70-75% of women at reproductive age will have at least one incident of VVC per lifetime and recurrence might occur in 40-50% [5]. The cottage cheese-like secretions are characteristic to VVC [6].
Trichomonas vaginalis is the causative agent of a rare vaginal infection called trichomonal vaginitis (TV), which is often accompanied by serious health consequences such as giving birth to premature infant and it elevates the chances of cervical cancer. It is characterized by frothy yellowish-green, foul-smelling discharge [7]. Aerobic vaginitis (AV) is a vaginal infection caused by large amounts of commensal aerobic bacteria originating from the intestine and it is markedly different from bacterial vaginosis [8]. AV is diagnosed by examining a wet film of vaginal discharge under a light microscope, combined with clinical manifestations. Generally, vaginitis is very common, precisely in low socio-economic level developing countries such as Egypt [9].
This study aimed to determine the prevalence of bacterial vaginosis, vulvovaginal Candidiasis, trichomonal vaginitis and aerobic vaginitis and their accompanied signs and symptoms in childbearing-age women in Egypt.
Methods
The study received the approval from the Ethics Committee of the Medical Research Institute (IORG#: IORG0008812), Alexandria University. An informed written consent was obtained from participating patients. It was conducted in accordance to the Helsinki Declaration for studies on human subjects in the period between October 2019 and October 2022.
A total of 1080 vaginal swabs were collected from female patients that were referred by gynecologists to Microbiology Laboratories for diagnosis of vaginal infections over the period of three years. All female patients were commonly suffering from any of the following symptoms: abnormal vaginal discharge, inflammation of the vagina and itching, painful intercourse and dysuria. Menstruating women or those who had received any antifungals or antibiotics in the past week or had performed vaginal douching in the preceding 24 hours were excluded from the study. Women were asked about most commonly encountered symptoms with vaginal infections like abnormal vaginal discharge, itching, painful sexual intercourse and inflammation.
Examination of the vagina
The vagina of each woman was examined for the vaginal discharge characteristics (odor, color, and consistency). Whiff test was done using 10 % potassium hydroxide (KOH) solution to test for the fishy odor [4]. Afterwards, a pH paper was applied to the lateral wall of the vagina to measure vaginal pH.
Sample collection for laboratory tests
Three dry cotton-tipped swabs were collected from vaginal walls while the patients were in the bladder lithotomy position. The vaginal swabs were then immediately sent to the nearby laboratories within approximately 30-40 minutes for further testing.
BV diagnosis
By referring to Amsel’s criteria, a BV positive diagnosis was confirmed if 3 of the following 4 signs were detected: 1) homogenous, thin, gray discharge; 2) whiff test positive; 3) pH >4.5 of the vaginal secretions; and 4) clue cells were found in wet mounts [10,11].
Nugent’s scores [12] were applied in which the relative abundance of large Gram-positive bacilli (lactobacilli), small Gram-variable rods (Gardnerella vaginalis), and curved Gram-variable rods (Mobiluncus) in Gram-stained vaginal smears were estimated by a semiquantitative evaluation method. Scores were interpreted as follows: 0–3 = normal, 4–6 = intermediate, and ≥7 = BV.
For detection of Gardnerella vaginalis in BV confirmed cases, a diagnostic rapid test was done using Liofilchem TM S. l R strips (Liofilchem, Italy) in which vaginal swabs were mixed thoroughly with 20 drops of the provided diluent for 1 minute according to the manufacturer’s instructions. This was followed by the removal of the swab and immersion of the test strip in the diluent for 10-15 minutes. Two purple lines indicated a positive test for the presence of Gardnerella spp. antigen (one for the control and the other for the antigen).
Diagnosis of VVC
Ten percent KOH wet mounts of the vaginal discharge were examined for detection of budding yeasts or pseudohyphae. This was followed by colony identification after cultivation of all samples on Sabouraud dextrose agar (SDA) and incubation for 48 hours at 37 °C [13].
Diagnosis of trichomonal vaginitis
Saline wet mounts of the vaginal secretions were examined for motile pear shaped trophozoites of T. vaginalis [14].
Identification of aerobic vaginitis
Wet smears were examined according to Donders et al. description criteria15 resulting in AV score interpreted as follows (<3 = no AV; 3–4 = light AV; 5–6 = moderate AV; and >6 = severe AV). Mounts were examined for the presence of parabasal cells, background flora, leucocytes, and toxic leucocytes. Vaginal swabs were streaked on Blood agar, MacConkey’s agar and chocolate agar and incubated for 24 hours at 37 °C for screening of aerobic bacterial vaginitis. Isolated bacteria were identified microscopically and phenotypically by routine biochemical identification tests.
Statistical analysis
Data were processed using SPSS (version 20, IBM Corp. USA). Pearson’s chi square test was used to detect relations between categorical variables, expressed as p value. All p values below 0.050 were interpreted to be statistically significant. Sensitivity, specificity, positive predictive value test and negative predictive value test of each criterion was determined individually.
Results
A total of 1080 females were enrolled in the study. The demographic characteristics of these patients are shown in Table 1. Most of them were middle age females (mean age: 30.6 ± 6 years). There was no statistically significant association between age and type of vaginal infection (p=0.130). Married women represented 85.7% of patients while divorced or widows were 14.3%, with also no significant association with any of the vaginal infections (p=0.261). Regarding the symptoms, there was a significant correlation between abnormal vaginal discharge and pregnancy (p=0.041). On the other hand, painful sexual intercourse was significantly higher in nonpregnant females (p=0.022). Vulvovaginal inflammation, itching and redness were significantly associated with VVC (p≤0.012).

Table 1.
Characteristics of childbearing-aged women included in this study (n=1080).
BV was the most prevalent single infection (43.8%). Distribution of other vaginal infections is shown in Table 2.

Table 2.
Prevalence of vaginal infections among the study group (n=1080).
Nugent’s score is the gold standard for diagnosis of BV. Of the 1080 vaginal swabs taken, 761 gave a Nugent’s score >7, providing a prevalence rate of 70.5% for BV (either single or mixed infection) – Figure 1. Regarding Amsel criteria, it was only able to diagnose 53% of BV. Therefore, the sensitivity of Amsel criteria was 73.5%, specificity was 95.9%, positive predictive value (PPV) was 97.7% and negative predictive value (NPV) was 60.35% (Table 3).
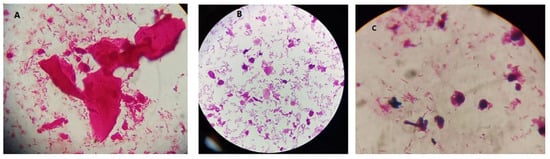
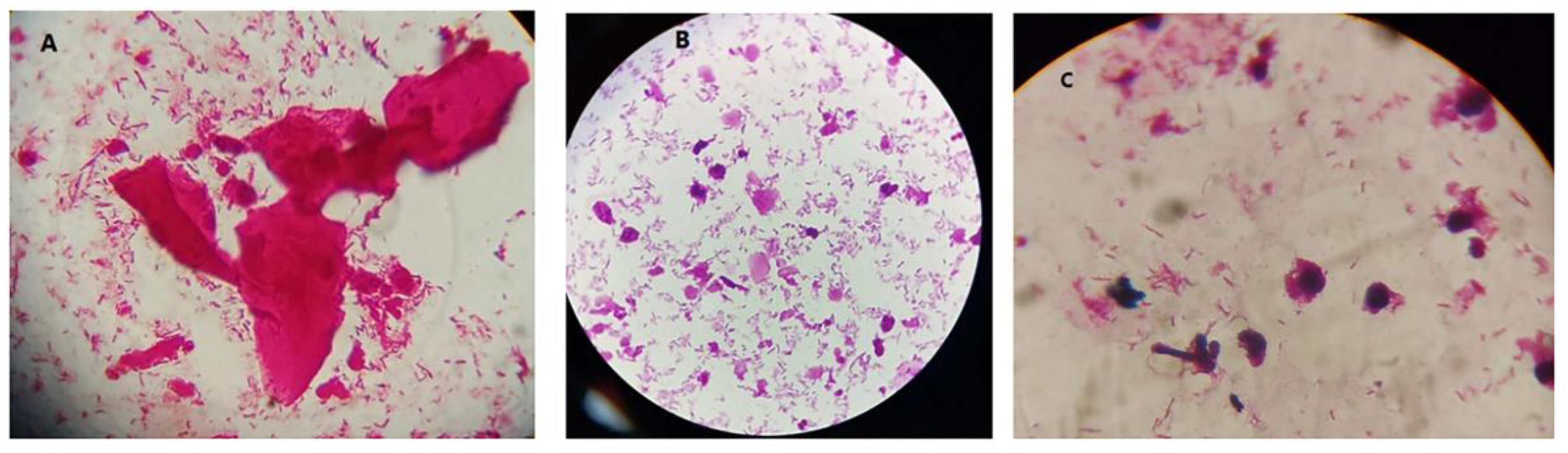
Figure 1.
Images for bacterial vaginosis. A: shows BV only. B and C show BV+ Candida. Some budding yeasts were noted in C.

Table 3.
Results of Amsel criteria compared to results of Nugent’s score (Gold standard) in the following contingency table (2 x 2).
All Amsel criteria together were detected only in 42% of females. The highest reported single criteria were homogenous discharge (58.2%), fishy odor (whiff test) (43.7%) and the detection of clue cells (39.9%). In terms of Nugent’s scores, most of the vaginal samples (70.5%) scored >7, interpreted as BV, whereas 19.2% scored 4–6 (intermediate), and 10.3% scored 1–3 (negative or normal).
The rapid diagnostic Liofilchem TM S. l R strips tests were used to detect the prevalence of Gardnerella vaginalis in BV confirmed cases, that were positive by both Nugent’s score and Amsel criteria. Out of the 560 cases, this test was done to only 100 cases. Gardnerella vaginalis was detected in 73% (73/100) of these BV cases (Figure 2). The sensitivity of this test was 73%.

Figure 2.
Rapid diagnostic strips for Gardnerella vaginalis. Two purple lines can be seen, one represents the control and the other shows a positive result for the presence of Gardnerella vaginalis.
VVC was assessed by both 10% KOH wet mounts and detection of colonial growth of Candida on SDA. Using the culture as a gold standard, the sensitivity of the KOH wet mount was found to be 77 % and the specificity was 96.4%.
AV was diagnosed by Donder’s criteria and traditional culture methods accompanied by biochemical reactions for identification of the pathogenic organisms. Upon examining the wet smears of the vaginal swabs, 21 (39.6%) samples scored 3–4 = light AV; 13 (24.5%) scored 5–6 = moderate AV; 19 (35.9%) scored >6 = severe AV. In regard to the bacterial isolates, E. coli represented 41.5%, Klebsiella spp. 24.5%, Enterococcus spp. 18.8%, Streptococcus spp. 11.4%, and Staphylococcus spp. 3.8% of the total AV (n=53). Four out of the 6 Streptococcus spp. were isolated from vaginal smears of pregnant females.
Discussion
Proper diagnosis of infectious vaginitis is always challenging. In addition to professional experience and scientific considerations, selecting an appropriate method for laboratory diagnosis requires contemplation of complexity, rate of un- or mis-interpretable specimens, and the cost.
However, many alternative diagnostic methods have been developed, such as the multiplex polymerase chain reaction (PCR) which can diagnose many pathogenic organisms including atypical bacteria that can cause infectious vaginitis. Yet, most of these diagnostic tools are very expensive and their specificities and sensitivities do not offer an enormous advantage over the conventional methods [16]. In addition, none of these advanced methods have the ability to cover all probable infectious agents. Therefore, traditional diagnostic methods are the most practical and affordable options for diagnosis, particularly in developing countries.
Given these considerations, this study aimed to study the prevalence of bacterial vaginosis, vulvovaginal candidiasis, trichomonal vaginitis and aerobic vaginitis in childbearing-age women in Egypt depending on conventional diagnostic methods due to limited resources.
The mean age of women suffering from vaginal infections in this study was 30.6 ± 6 years. Similar mean age was stated in several other studies [9,17,18,19]. Hereby, it is well-recognized that vaginal infections are always detected in middle-aged women in reproductive age.
Clinical criteria alone always fail to diagnose BV because its components are subjective and depend on the skills of the clinician and equipment availability. Nugent’s score and Amsel criteria remain the most reliable tools for BV diagnosis. BV showed a high prevalence in this study, whether as a single infection (43.8%) or mixed with VVC (26.6%). This was followed by VVC as a single infection (24.2%). Consistent with our results, a study conducted in Yemen reported that BV was the most frequent single infection and it was followed by VVC [20]. An Egyptian study as well showed collateral results in which BV prevalence was 59.1% and VVC was 50.2% [18].
Nevertheless, a cross sectional study which was carried out in upper Egypt, reported that VVC was the most prevalent infection (60.8%) while 37.1% were diagnosed with BV [9]. This can be attributed to variations in subtypes of different populations. Moreover, the actual prevalence may be higher since not less than 30% of BV patients are not diagnosed [21], which may illustrate the lower detecting power of Amsel criteria (which uses clinical signs which are not standardized) in comparison to that of Nugent’s score.
In this study, the sensitivity of Amsel criteria was found to be 73.5% in which we considered Nugent’s method as our gold standard. In agreement with our results, an Indian study found the sensitivity of Amsel criteria to be 66.67%. This low sensitivity of Amsel’s criteria was also reported by Centers of Disease Control and Prevention (CDC) in sexually transmitted infections treatment guidelines 2021, in which they reported the sensitivity and specificity of the Amsel criteria to be 37-70% and 94-99%, respectively, in comparison to the Nugent score [22]. In spite of the lower sensitivity of Amsel criteria, a high specificity was reported for Amsel criteria (94.7%) that is in line with the reported values of specificity in other studies [17,23]. Our study suggests that Amsel criteria may not be very reliable in diagnosis of BV.
Moreover, the rapid test that can detect Gardnerella vaginalis cannot be used routinely in low-income countries such as Egypt due to its high cost. There is a huge demand for a low-cost, reliable diagnostic technique that combines clinical and microbiological parameters to increase sensitivity while maintaining specificity for diagnosis of BV.
Trichomonal vaginitis was the lowest prevalent infection among our study group (0.5%). This low prevalence was also revealed by other studies [9,18,20]. This can be explained by the low sensitivity of the microscopic examination (44%-68%) in the diagnosis of trichomonal vaginitis when compared to culture, as reported by CDC sexually transmitted infections treatment guideline, 2021 [22].
Aerobic vaginitis also showed a low prevalence (4.9%). The prevalent bacterial isolates detected were E. coli (41.5%), followed by Klebsiella spp. and Enterococcus spp. (18.8%). These isolates are considered to be part of the lower gastrointestinal normal flora, thus can be considered as an ascending infection which results from bad hygienic practices. Overlapping results regarding the grades of aerobic vaginitis and the type of isolated bacteria were reported by Rumyantseva et al. and Ma Xiaotong et al [19,24].
Professional microbiologists are always demanded in order to accurately diagnose aerobic vaginitis depending on microscopic examination and interpretation of culture plates in order not to misdiagnose contaminated vaginal samples as aerobic vaginitis.
The strength of the current study relies on the large sample size and the use of simple diagnostic tools which can be easily applied in resources-limited developing countries. On the other hand, its limitations include the use of the microscopic examination only for the detection trichomonal vaginitis, which in turn revealed very low prevalence of this type of infection. Moreover, the rapid diagnostic Liofilchem TM S. l R strips test was only performed on 100 samples due to its high cost.
Conclusions
The majority of studies indicate that women who are at reproductive age are more likely to contract vaginal infections, especially bacterial vaginosis. BV showed the highest prevalence, followed by VVC in this study. Mixed infections between BV and VVC were evidently noted, therefore good reliable microbiology results are always needed for effective treatment. Aerobic vaginitis showed low prevalence. Streptococcus spp. were mostly isolated from pregnant females. The least recognized infectious vaginitis was trichomonal vaginitis. The low prevalence of Trichomonas vaginalis may be due to the dependance on microscopic diagnosis, and thus more advanced diagnostic tools are required.
Author Contributions
SR: practical microbiology diagnostics, data collection, draft manuscript preparation and final revision. OA: practical work and writing the draft manuscript. All authors read and approved the final version of the manuscript.
Funding
None to declare.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
All authors – none to declare.
References
- Omosa-Manyonyi, G.S.; Koyio, L.N.; Mwangi, E.W.; Gathura, H.; van der Ven, A.; Oever, J.T. Inadequacies in service delivery for the diagnosis and treatment of vaginitis and vaginosis in Nairobi. Kenya. Int J STD AIDS. 2022, 33, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Loveless, M.; Myint, O. Vulvovaginitis-presentation of more common problems in pediatric and adolescent gynecology. Best Pract Res Clin Obstet Gynaecol. 2018, 48, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Nigam, N.; Goel, R.; Goel, J.; Shukla, M. Etiopathogenesis of vaginal discharge among married women in reproductive age group residing in rural area of Bhojipura District, Western Uttar Pradesh, India. Int J Reprod Contracept Obstet Gynecol. 2019, 8, 2599. [Google Scholar] [CrossRef]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef]
- Hamad, M.; Kazandji, N.; Awadallah, S.; Allam, H. Prevalence and epidemiological characteristics of vaginal candidiasis in the UAE. Mycoses. 2014, 57, 184–190. [Google Scholar] [CrossRef]
- Pustan, L.; Ailiesei, O.; Dunca, S. Trichomonas vaginalis a risk factor for cervical cancer. J Exp Mol Biol. 2010, 11, 107–112. [Google Scholar]
- Kaambo, E.; Africa, C.; Chambuso, R.; Passmore, J.S. Vaginal microbiomes associated with aerobic vaginitis and bacterial vaginosis. Front Public Health. 2018, 6, 78. [Google Scholar] [CrossRef]
- Abbas, A.M.; Shaaban, O.M.; Badran, S.M.; Shaltout, A.S.; Nasr, A.; Abdullah, S.A. Risk factors and health hazards of vaginal infections in upper Egypt: A cross sectional study. Thai J Obstet Gynaecol. 2016, 50–56. [Google Scholar]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Tal, R.; Seifer, D.B. Ovarian reserve testing: A user's guide. Am J Obstet Gynecol. 2017, 217, 129–140. [Google Scholar] [CrossRef]
- Delaney, M.L.; Onderdonk, A.B. Nugent score related to vaginal culture in pregnant women. Obstet Gynecol. 2001, 98, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bitew, A.; Abebaw, Y. Vulvovaginal candidiasis: Species distribution of Candida and their antifungal susceptibility pattern. BMC Women's Health. 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.R.; Super, H.; Fripp, P.J. Comparison of four techniques for the routine diagnosis of Trichomonas vaginalis infection. J Clin Pathol. 1976, 29, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Vereecken, A.; Bosmans, E.; Dekeersmaecker, A.; Salembier, G.; Spitz, B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: Aerobic vaginitis. BJOG. 2002, 109, 34–43. [Google Scholar] [CrossRef]
- Verstraelen, H.; Verhelst, R. Bacterial vaginosis: An update on diagnosis and treatment. Expert Rev Anti Infect Ther. 2009, 7, 1109–1124. [Google Scholar] [CrossRef]
- Modak, T.; Arora, P.; Agnes, C.; et al. Diagnosis of bacterial vaginosis in cases of abnormal vaginal discharge: Comparison of clinical and microbiological criteria. J Infect Dev Ctries. 2011, 5, 353–360. [Google Scholar] [CrossRef]
- Shawaky, S.M.; Al Shammari, M.M.A.; Sewelliam, M.S.; Ghazal, A.A.E.R.; Amer, A.N. A study on vaginitis among pregnant and non-pregnant females in Alexandria, Egypt: An unexpected high rate of mixed vaginal infection. AIMS Microbiol. 2022, 8, 167–177. [Google Scholar] [CrossRef]
- Rumyantseva, T.A.; Bellen, G.; Savochkina, Y.A.; Guschin, A.E.; Donders, G.G. Diagnosis of aerobic vaginitis by quantitative real-time PCR. Arch Gynecol Obstet. 2016, 294, 109–114. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.; Mahdy, M.A.K.; Abdul-Ghani, R.; et al. Bacterial vaginosis, vulvovaginal candidiasis and trichomonal vaginitis among reproductive-aged women seeking primary healthcare in Sana'a city, Yemen. BMC Infect Dis. 2019, 19, 879. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Morton, A.N.; Garland, S.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005, 43, 1304–1308. [Google Scholar] [CrossRef]
- Hazra, A.; Collison, M.W.; Davis, A.M. CDC sexually transmitted infections treatment guidelines, 2021. JAMA. 2022, 327, 870–871. [Google Scholar] [CrossRef]
- Sha, B.E.; Zariffard, M.R.; Wang, Q.J.; et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005, 191, 25–32. [Google Scholar] [CrossRef]
- Ma, X.; Wu, M.; Wang, C.; et al. The pathogenesis of prevalent aerobic bacteria in aerobic vaginitis and adverse pregnancy outcomes: A narrative review. Reprod Health. 2022, 19, 21. [Google Scholar] [CrossRef]
© GERMS 2023.