Introduction
Pityriasis versicolor (PV) is a highly prevalent chronic superficial skin fungal infection caused by a lipophilic fungus of
Malassezia species in tropical areas [
1,
2,
3,
4,
5]. Factors that play a role in this infection are warm temperature, humidity, hyperhidrosis, oral contraception, oral corticosteroid, Cushing disease, immunosuppressive agents, pregnancy, and malnutrition [
6]. The diagnosis of PV is established by medical history, physical examination, and microscopic examination [
1,
3]. The typical lesions of PV include macule, plaque, and follicular papule of varying color with a thin scale above the lesion [
3,
7]. Scale is frequently invisible, therefore, it is necessary to perform a scale provocation test, known as fingernail sign or evoked scale sign [
8]. Positive results of 10-20% potassium hydroxide (KOH) and Parker Blue-Black
® ink will reveal short hyphae and clusters of spores, which appear as “spaghetti and meatballs” [
1,
3,
9]. Wood’s Lamp could add a visualization of the infected lesion and guide the location to do the skin smea [
9].
The topical antifungal drug has been reported as the first-line therapy, while the systemic antifungal drug is recommended for relapsed disease or diseases that are non-responsive to topical treatment [
10,
11]. In topical therapy, in addition to the active substances, the form of drugs is also important to be taken into consideration, such as shampoo, cream, or solution. Cream could be used for limited lesions, whereas for more extended and generalized lesions, shampoo or solution are more convenient to use [
10]. Detergent-based shampoo has advantages because of its mild irritative effect [
12], that causes the upper part of the stratum corneum to peel and the antifungal substance to be easily absorbed. Treatment of PV usually requires 2-3 weeks [
10,
12]. Unfortunately, the recurrence rate of PV is high, around 60-80% within 2 years after treatment [
11,
13].
An open-label trial by Rathi et al. [
14] involving 30 subjects reported good efficacy in removing lesions and PV symptoms with 2% ketoconazole shampoo, 10 minutes, daily, application for 3 days. Aggarwal et al. [
15] reported that the cure rate of 2% ketoconazole shampoo treatment in patients with PV was 95%, and the cure rate of 2.5% selenium sulfide shampoo was 85%, in which each group received an application of shampoo for 5 minutes, once every week for 3 weeks, and the efficacy of both shampoos was not significantly different [
15].
In Indonesia, 2.5% selenium sulfide is available only as a lotion, while the shampoo is only containing 1.8% selenium sulfide and is indicated for antidandruff treatment. There has been no study comparing the efficacy and safety of 1.8% selenium sulfide shampoo and 2% ketoconazole shampoo. In this study, the efficacy of PV treatment is assessed by mycological examination, and the safety of treatment is assessed by subjective and objective data.
Methods
This was a two-arms double-blind randomized clinical trial in patients with PV. The participants were randomly assigned following block randomization procedure to receive 1.8% selenium sulfide shampoo or 2% ketoconazole shampoo. Both shampoos were packed in similar and non-transparent package and were given a study serial number. The amount of shampoo was equal, 10 mL. This study was conducted in the Pulo Gadung District Public Health Center, Jakarta, Indonesia, in September–December 2018. Subjects were recruited by consecutive sampling.
The inclusion criteria were subjects aged 12-60 years old with PV, which is definedas PV-associated skin lesion and with 20% KOH and Parker Blue-Black® ink examination showing short hyphae combined with or without a cluster of spores; written informed consent for adults and, for subjects aged below 18 years old should have written or verbal informed consent from one or both parents. The exclusion criteria were history of a hypersensitivity to the shampoos that were being tested, impaired skin integrity, being under topical antifungal treatment of less than 2 weeks or systemic treatment of less than 1 month, pregnancy or lactation, and PV lesion on the face. We calculated the sample size using a formula to compare the proportions of two arms.
Medical interview, physical examination, scale provocation test, Wood lamp examination, and KOH—Parker Blue-Black® ink staining were performed. Wood lamp examination was performed in a dark area and the positive Wood lamp was defined by the two most active lesions seen as yellow-green fluorescence appearance. The positive scales were directly visualized by the eye, meanwhile, positive scale provocation test was defined as a positive scale seen after provocation by an object glass. Subjects with positive KOH—Parker Blue-Black® staining were treated with 1.8%, SeS2, or 2% ketoconazole for 7-14 days according to random allocation. In this study, a cure was defined as a negative KOH—Parker Blue-Black® smear result. For subjects with negative KOH—Parker Blue-Black® smear on day 7, treatment was terminated. As for subjects with positive KOH—Parker Blue-Black® smear on day 7, treatment was continued until day 14. If the KOH—Parker Blue-Black® was still positive on day 14, the subject was allocated to the treatment failure group and treated with systemic antifungal treatment. The subject who did not complete the treatment course was considered treatment failure according to the intention to treat analysis. Two samples were taken in each KOH—Parker Blue-Black® staining procedure.
The side effects of treatment were assessed by subjective and objective measures on day 7 and 14. Subjective side effects assessment was performed by symptoms expressed by subjects directly to the investigator or by a record in the daily log participant’s book. The objective side effects assessment was done by skin examination of signs of inflammation. Only treatment-related locations of side effects were included in the analysis. In this study, itchiness that was considered a side effect was companied by a burning and tingling sensation.
Approval to the study protocol was provided by the Ethics Committees at the Faculty of Medicine Universitas Indonesia—Dr. Cipto Mangunkusumo Hospital (Number: 0760//UN2.F1/ETIK/2018). All participants provided written informed consent before enrollment. This study protocol has been registered at ClinicalTrial.gov (registration number: NCT04007237).
Normally distributed numerical variables are presented as mean and standard deviation. Nominal variables are presented as frequency and percentage. Categorical variables were subject to the Chi-square test. Confidence of interval (CI) and relative risk (RR) are presented in the statistical analysis. Analysis of study results data was performed using SPSS program version 20 (IBM Corp., USA). Statistically significant was defined by p-value <0.05. Intention to treat analysis was performed in this study.
Results
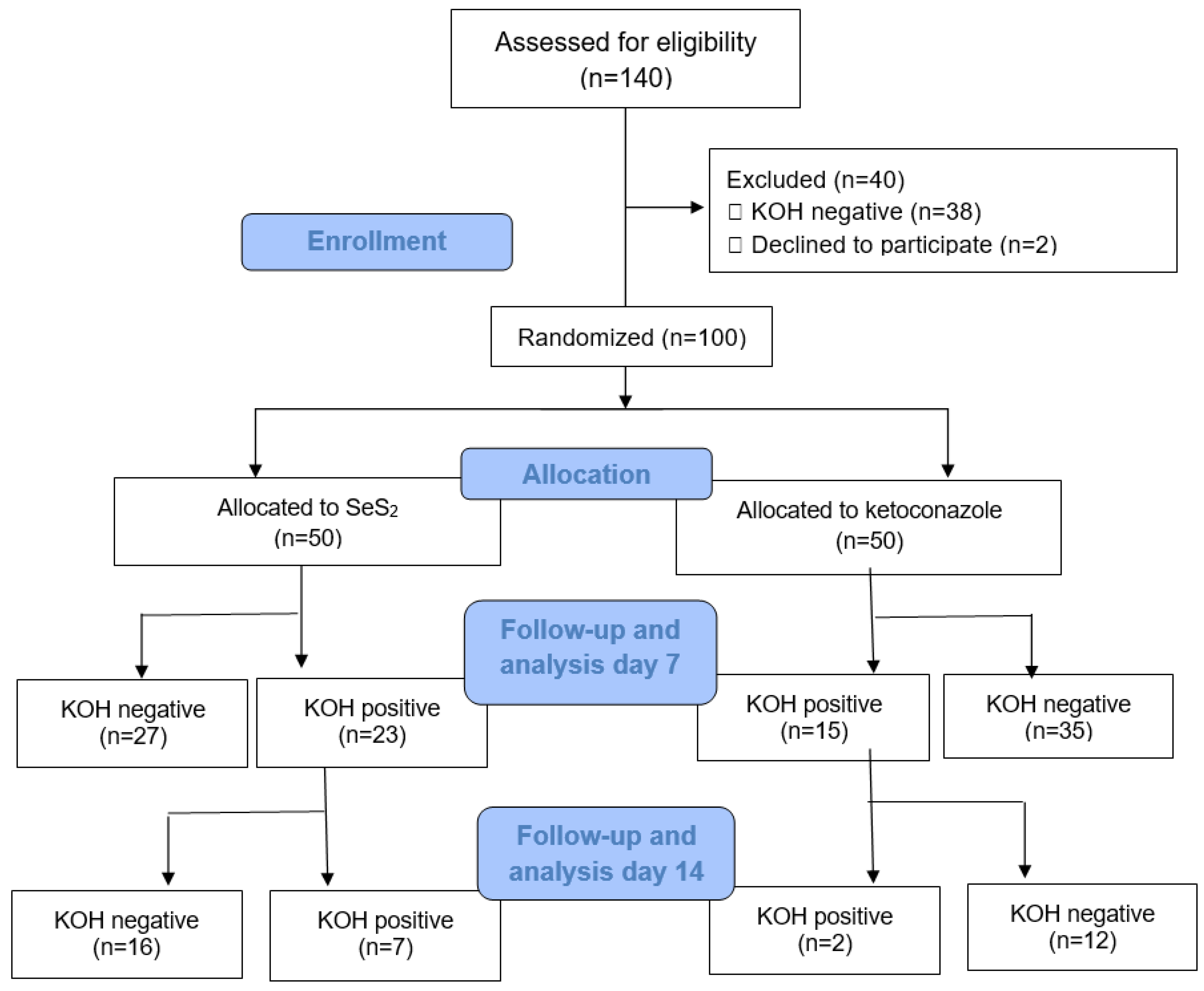
From 100 subjects that fulfilled the inclusion and exclusion criteria and were randomized, 50 subjects were treated with 1.8% SeS
2, and 50 subjects were treated with 2% ketoconazole. The number of subjects that completed the treatment course was 99 subjects and this number is still above the minimal number of subjects required for analysis of mycological efficacy, i.e., 98 subjects. The study flow is described by a CONSORT diagram (
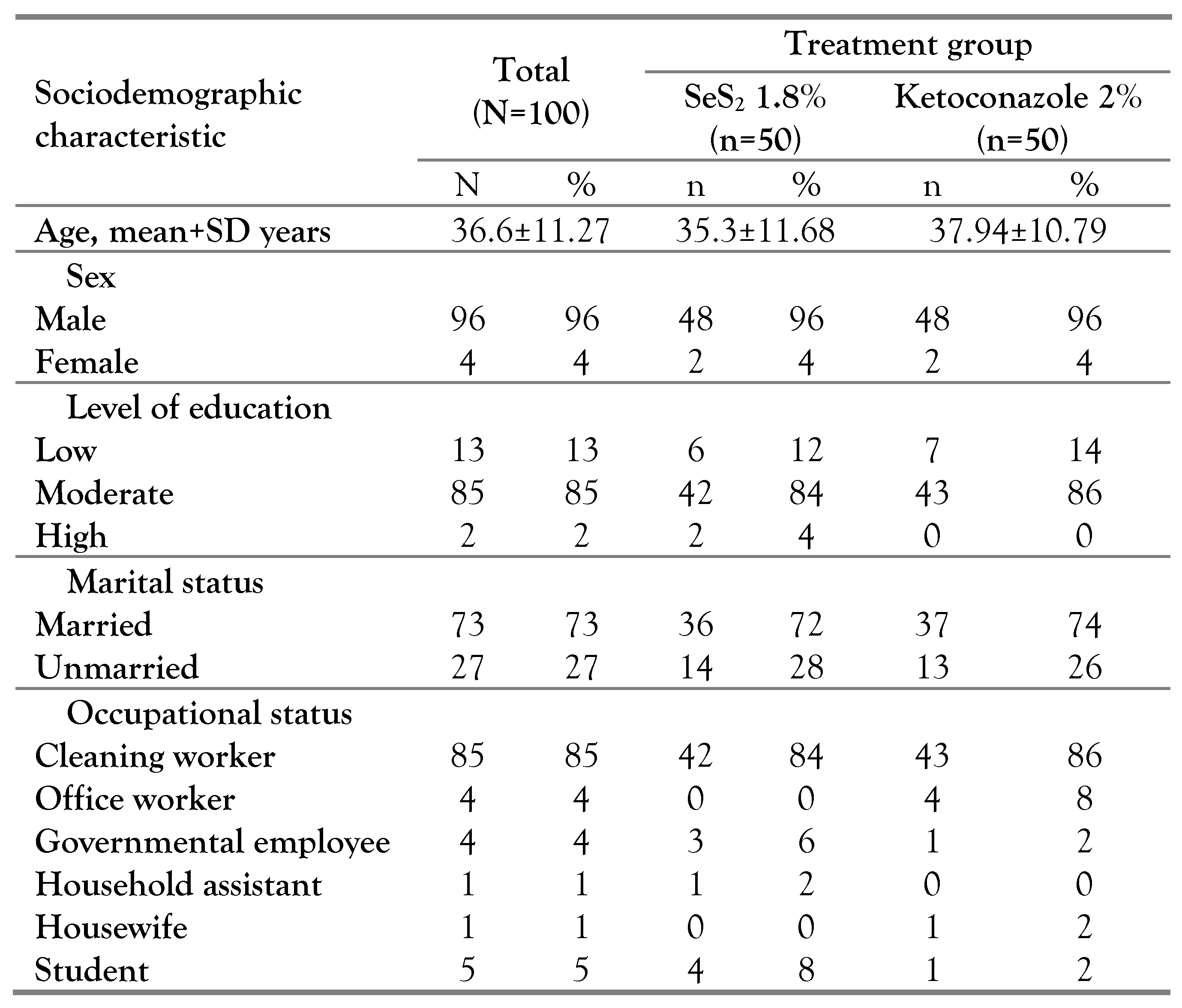
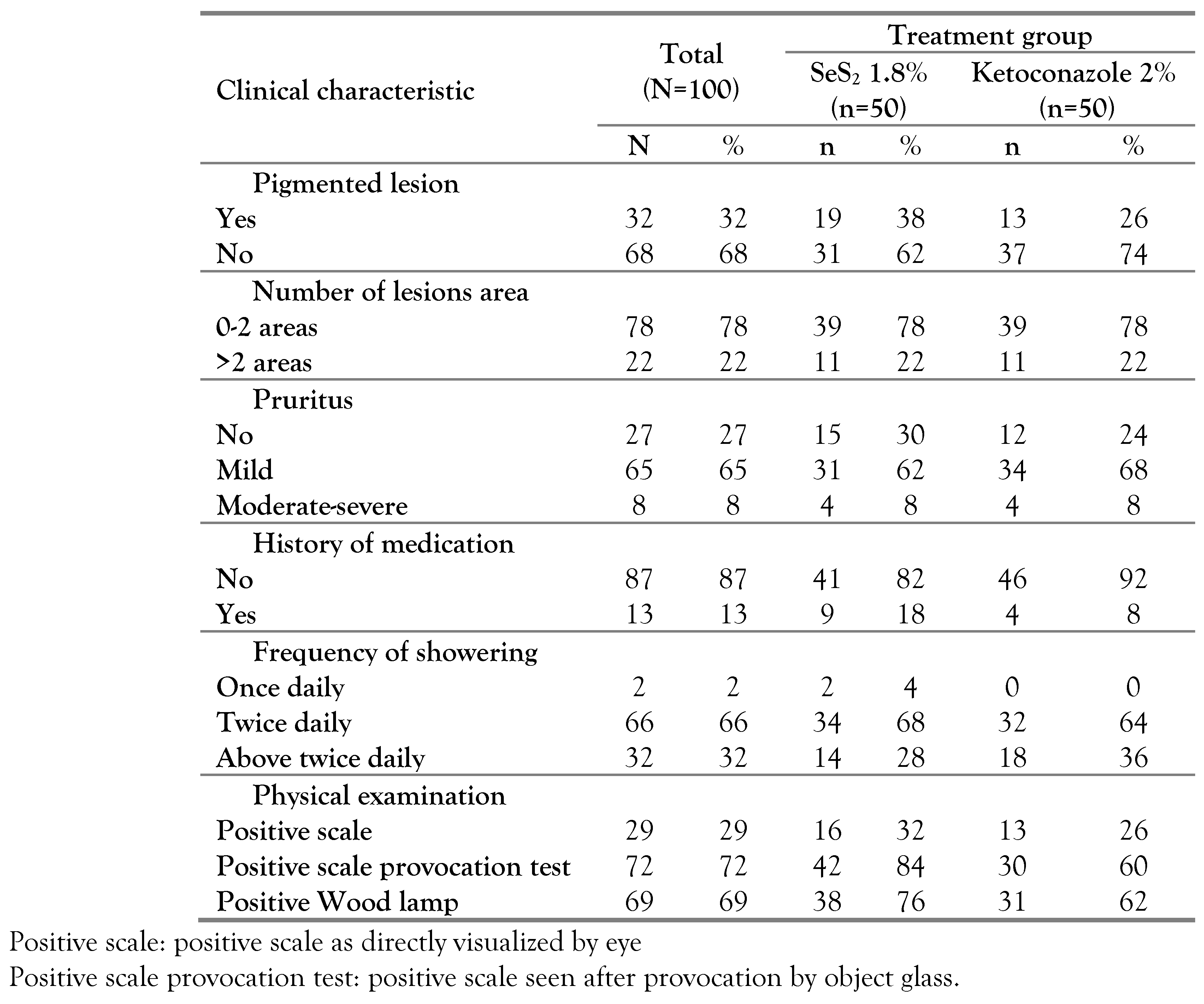
Figure 1), meanwhile subjects’ sociodemographic characteristics and baseline clinical characteristics are described in
Table 1 and
Table 2, respectively.
Most of the subjects had hypopigmented PV lesions, located in the trunk as well as other locations including the arm, shoulder, neck, abdomen, and waist. Furthermore, positive thin scales by provocation test and positive Wood lamp were observed in most of the subjects (
Table 2).
We first determined the mycological efficacy. As described in
Table 3, there was a 54% negative result in the selenium group and a 70% negative result in the ketoconazole group on day 7, however, this difference did not reach the statistical threshold (RR=1.5 [95%CI 0.9-2.5], p=0.099). Subjects with negative KOH—Parker Blue-Black
® smear on day 7 were classified as cured.
After the course of treatment on day 14, there were 86% and 94% of subjects in the selenium arm and in the ketoconazole arm, respectively, who showed negative KOH—Parker Blue-Black® smear. Statistical analysis showed that this difference is not significant (RR=2.3 [95%CI 0.6-8.5] and p=0.182).
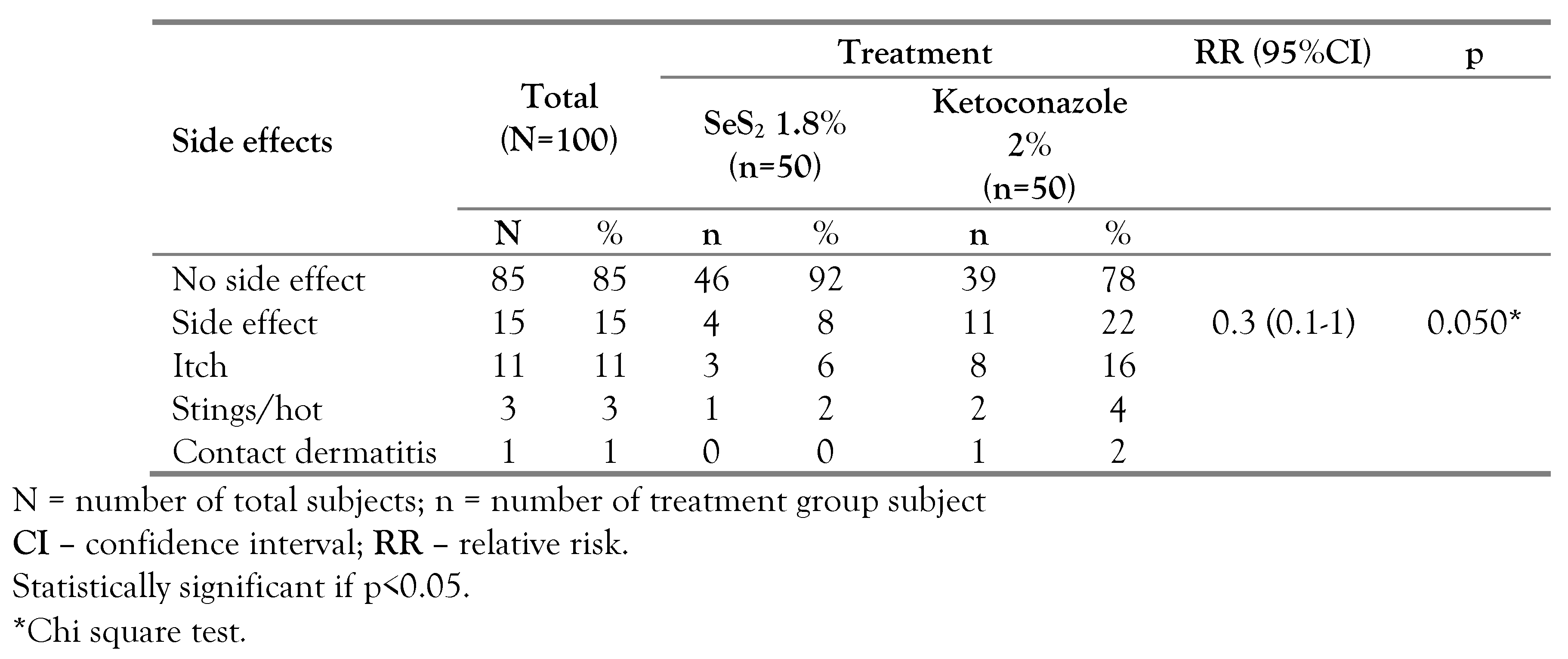
On day 7, side effects were more frequent in the ketoconazole group as compared to the selenium group (22% vs. 8%) as shown in
Table 4. In the selenium group, 3 subjects had itchiness and 1 subject felt a tingling sensation. In the ketoconazole group, 2 subjects had a burning and tingling sensation, when using the shampoo and 1 subject showed a contact dermatitis lesion. Pruritus was mostly mild and spontaneously resolved several minutes after using the shampoo. Side effect evaluation on day 14 was performed in 37 subjects who continued the treatment; 13% subjects in the selenium group and 21.4% subjects in the ketoconazole group felt itchiness (
Table 5).
Discussion
This was a double-blind randomized controlled trial and subjects were divided into selenium and ketoconazole arms by block randomization. The sample size of the study is sufficient, 100 subjects, with quite a similar economical background, and 85% of the subjects had the same occupation, therefore the environmental factors that may confound the PV could be managed. In this study, we compared the mycological efficacy and safety of SeS2 1.8% and ketoconazole 2% shampoo for the treatment of PV.
Our subjects’ mean age was slightly higher than the study by Heidrich et al. [
16] in Brazil and quite similar to subjects in the study by Krisanty et al. [
2] in Indonesia. A previous report by Kyriakis et al. [
17] suggests that in the tropical region, the incidence of PV was more dominant in males. Our study subjects did not represent the general patients with PV in Jakarta, Indonesia, because most of the subjects were recruited from male-dominant workplaces. The level of education is an important factor that may influence disease understanding and treatment compliance.
Factors that are related to PV pathogenesis are high temperature and humidity, reduced evaporation due to long-term clothes, and changes in sebum composition, which induce morphological change of
Malassezia spp. to become mycelium, causing the formation of PV lesion [
18].
Most of the subjects did not have symptoms, while some subjects complained of having pigmented lesions with no itchy sensation. This may contribute to subjects not seeking medical treatment in the healthcare facility. However, a study in Nigeria found that 45% of patients with signs of PV eventually visited healthcare facilities [
19].
By scale provocation test, positive thin scales were found in most of the subjects. Scale provocation test is an important diagnostic procedure to establish the diagnosis of PV, especially when a mycological test is not available [
8,
20]. False negative results could be obtained if patients with PV had just finished showering or the lesion has been treated [
21].
On Wood’s lamp examination, most of the subjects had positive result. This finding is lower as compared to a study by Durdu et al. who reported that 100% of patients with PV showed yellow-green fluorescence by Wood lamp examination that suggests positive result [
22]. Lower positivity rate by Wood’s lamp in this study may be because the skin lesion examination was performed in the morning when subjects had just finished showering. Showering may result in pale color observed under Wood’s lamp because of diluted pigment from fungi that came in contact with water [
23].
Itch and scales during the treatment period might be due to active PV lesions or because of mild irritation from the active substances in the shampoo. Mild irritation could increase the epidermis turnover, resulting in desquamation. These conditions make the subjective itchy symptoms and scales difficult as treatment indicators.
Although it did not reach a statistical threshold, we found that the mycological efficacy of ketoconazole was superior in the 7-day assessment, however, if the treatment is continued until 14 days the efficacy of both treatments was not different significantly. A similar result was observed by Aggarwal et al., in which the treatment success rate for selenium sulfide 2.5% and ketoconazole 2% were 85% and 95%, respectively, for three weeks treatment, once a week and 5 minutes each [
15]. The findings suggest the potential of selenium sulfide as an alternative treatment for PV.
Side effects of selenium sulfide include pruritus, allergic and irritant contact dermatitis, burning sensation, tingling sensation, garlic breath, hair and nail discoloration, alopecia, and suppressed lactation [
24]. Meanwhile, the side effects of ketoconazole 2% that have been reported are mild irritation and pruritus [
14]. In this study, the side effect rate in the ketoconazole 2% arm was higher than in the SeS
2 1.8% arm. Rathi et al. [
14] reported that two of 30 subjects had an itchy complaint and mild irritation under the ketoconazole 2% treatment of daily 10 minutes application for 3 days. However, Aggarwal et al. [
15] reported no side effects among subjects with ketoconazole 2% or selenium sulfide 2.5% once a week, 5 minutes application, 3 weeks duration.
Both selenium sulfide shampoo and ketoconazole shampoo had several advantages and disadvantages. Ketoconazole was able to meet its therapeutic target in terms of negative fungal staining faster than selenium sulfide. Moreover, ketoconazole had lotion preparation that allows the application in sensitive skin areas such as the genital area and can be used in children [
25]. Selenium sulfide is more efficient in terms of price and easy to apply [
12]. Furthermore, our study found that there is a tendency for the side effects of selenium sulfide to be less frequent. Finally, selenium sulfide could be used as an option to reduce the emergence of ketoconazole resistance in fungal infection.
Limitations of the study include: perfect obscurity of the test material is quite difficult because selenium sulfide shampoo is orange in color and slightly more fragrant, while ketoconazole shampoo is pink. Efforts made by researchers were to pack shampoo bottles in brown and opaque wrappers, as well as an explanation to participants at the beginning of the study that the color of the drug varies from orange to pink and can be slightly more fragrant or pungent. In this study, there was one participant who did not continue treatment on the 14th day visit, but the participant number still met the minimum required number of participants.
Conclusions
There were no significant differences in terms of mycological efficacy and side effects between selenium sulfide 1.8% shampoo and ketoconazole 2% shampoo for the treatment of PV. Selenium sulfide 1.8% could be considered as one of the options in the treatment of PV, because of mycological efficacy, being cheaper and having minimum side effects. In this chronic recurrent disease, the use of selenium sulfide shampoo as maintenance therapy can be expected to be more economical. Further study that reveals the duration of shampoo application for PV treatment, with various application routes is needed.
Author Contributions
L contributed in conceptualization; methodology; data collection; formal analysis; validation; and writing, review, editing, and approval of the final manuscript. LS contributed in conceptualization, methodology, formal analysis, validation, and approval of the final manuscript. SRFS contributed in conceptualization, methodology, formal analysis, validation, investigation, and approval of the final manuscript. TL contributed in conceptualization, methodology, formal analysis, validation, investigation, and approval of the final manuscript. KB contributed in conceptualization, methodology, formal analysis, validation, investigation, and approval of the final manuscript. All authors read and approved the final version of the manuscript.
Clinical trial registration number
NCT04007237.
Institutional Review Board Statement
This study has been approved by the Institutional Review Board (IRB) of Faculty of Medicine Universitas Indonesia (Number: 0760//UN2.F1/ETIK/2018).
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
We highly appreciated all medical and non-medical staff at the Public Health Center (Pusat Kesehatan Masyarakat) Kecamatan Pulo Gadung, Jakarta, Indonesia. The investigators acknowledge the support from Rohto Laboratories Indonesia who provided Selsun shampoo in this study.
Conflicts of interest
All authors—none to declare.
References
- Kundu, R.V.; Garg, A. Yeast infections: candidiasis, tinea (pityriasis) versicolor, and Malassezia (Pityrosporum) Folliculitis. In Fitzpatrick’s dermatology in general medicine, 9th ed.; Goldsmith, L.A., Katz, S.I., Gilchrest, B.A., Paller, A.S., Leffel, J.D., Eds.; McGraw-Hill: New York, 2019; pp. 2307–2310. [Google Scholar]
- Krisanty, R.I.; Bramono, K.; Made Wisnu, I. Identification of Malassezia species from pityriasis versicolor in Indonesia and its relationship with clinical characteristics. Mycoses. 2009, 52, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Karray, M.; McKinney, W.P. Tinea versicolor. [Updated 2021 Aug 11]. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2022; Available from: https://www.ncbi.nlm.nih.gov/books/NBK482500/. [Google Scholar]
- Hu, S.W.; Bigby, M. Pityriasis versicolor: a systematic review of interventions. Arch Dermatol. 2010, 146, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Meixner, J.A.; Smith, M.B.; McGinnis, M.R. Superficial mycoses and dermatophytoses. In Tropical dermatology; Tyring, S.K., Lupi, O., Hengge, U.R., Eds.; Elsevier Inc.: Philadelphia, 2006; pp. 186–187. [Google Scholar] [CrossRef]
- Mellen, L.A.; Vallee, J.; Feldman, S.R.; Fleischer, A.B., Jr. Treatment of pityriasis versicolor in the United States. J Dermatolog Treat. 2004, 15, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Levin, N.A.; Delano, S. Evaluation and treatment of Malassezia-related skin disorders. Cosmet Dematol. 2011, 24, 137–145. [Google Scholar]
- Shi, V.S.; Lio, P.A. Diagnosis of pityriasis versicolor in paediatrics: the evoked scale sign. Arch Dis Child. 2011, 96, 392–393. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.A.; Dinehart, S.M.; Farmer, E.R.; et al. Guidelines of care for superficial mycotic infections of the skin: Pityriasis (tinea) versicolor. J Am Acad Dermatol. 1996, 34, P287–P289. [Google Scholar] [CrossRef] [PubMed]
- Radiono, S.; Suyoso, S.; Bramono, S. Pitiriasis Versikolor. In Dermatomikosis Superfisialis; Bramono, K., Suyoso, S., Indriatmi, W., Ramali, L.M., Widaty, S., Ervianty, E., Eds.; Universitas Indonesia Faculty of Medicine Publishing: Jakarta, 2013; pp. 24–34. [Google Scholar]
- Gupta, A.K.; Folley, K.A. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015, 1, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Ashbee, H.R. Mycology. In Rook’s textbook of dermatology, 8th Edition; Burns, T., Breathnach, S., Cox, N., Griffiths, C., Eds.; Wiley-Blackwell: Oxford, 2010; Volume 36, pp. 10–12. [Google Scholar]
- Kallini, J.R.; Riaz, F.; Khachemoune, A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014, 53, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.K. Ketoconazole 2% shampoo in pityriasis versicolor: An open trial. Indian J Dermatol Venereol Leprol. 2003, 69, 142–143, https://ijdvl.com/ketoconazole-2-shampoo-in-pityriasis-versicolor-an-open-trial/. [Google Scholar] [PubMed]
- Aggarwal, K.; Jain, V.K.; Sangwan, S. Comparative study of ketoconazole versus selenium sulfide shampoo in pityriasis versicolor. Indian J Dermatol Venereol Leprol. 2003, 69, 86–87. [Google Scholar] [PubMed]
- Heidrich, D.; Daboit, T.C.; Stopiglia, C.D.; et al. Sixteen years of pityriasis versicolor in metropolitan area of Porto Alegre, Southern Brazil. Rev Inst Med Trop Sao Paulo. 2015, 57, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, K.P.; Terzoudi, S.; Palamaras, I.; Pagana, G.; Michailides, C.; Emmanuelides, S. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006, 49, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Morais, P.M.; Cunha Mda, G.; Frota, M.Z. Clinical aspects of patients with pityriasis versicolor seen at a referral center for tropical dermatology in Manaus, Amazonas, Brazil. An Bras Dermatol. 2010, 85, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Yahya, H. Knowledge, perception, and practice of patients about pityriasis versicolor in Kaduna, North Central Nigeria. Int J Dermatol. 2017, 56, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Magiatis, P.; Hantschke, M.; Bassukas, I.D.; Velegraki, A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012, 25, 106–141. [Google Scholar] [CrossRef] [PubMed]
- Kangle, S.; Amladi, S.; Sawant, S. Scaly signs in dermatology. Indian J Dermatol Venereol Leprol. 2006, 72, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Durdu, M.; Güran, M.; Ilkit, M. Epidemiological characteristics of Malassezia folliculitis and use of the May-Grunwald-Giemsa stain to diagnose the infection. Diagn Microbiol Infect Dis. 2013, 76, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, A. Robert Williams Wood: pioneer of invisible light. Photodermatol Photoimmunol Photomed. 2016, 32, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.C.; Nickson, R.M.; Waring, R.H. The biological activity of selenium sulfide. Sulfur Rep. 1993, 13, 279–289. [Google Scholar] [CrossRef]
- Antifungal agents for common paediatric infections. Can J Infect Dis Med Microbiol. 2008, 19, 15–18. [CrossRef]