Association Between Vitamin D Receptor Gene Polymorphisms and Fibrosis Susceptibility in Greek Patients with HCV Infection
Abstract
Introduction
Methods
Patient characteristics
DNA isolation and genotyping of ApaI and TaqI polymorphisms
Statistical analysis
Analysis of Hardy-Weinberg Equilibrium
Results
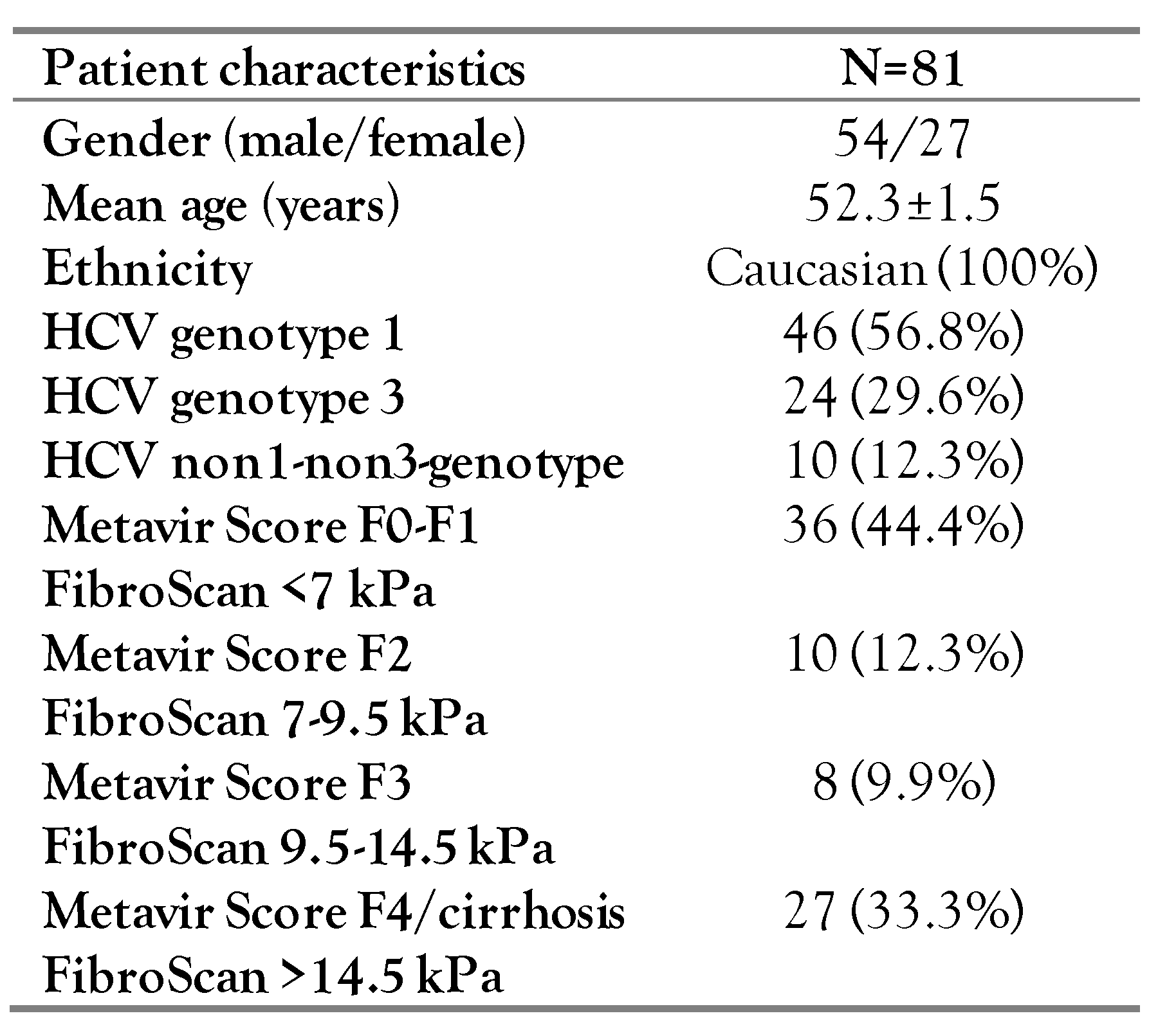
Patient characteristics
Hardy-Weinberg Equilibrium and Linkage Disequilibrium
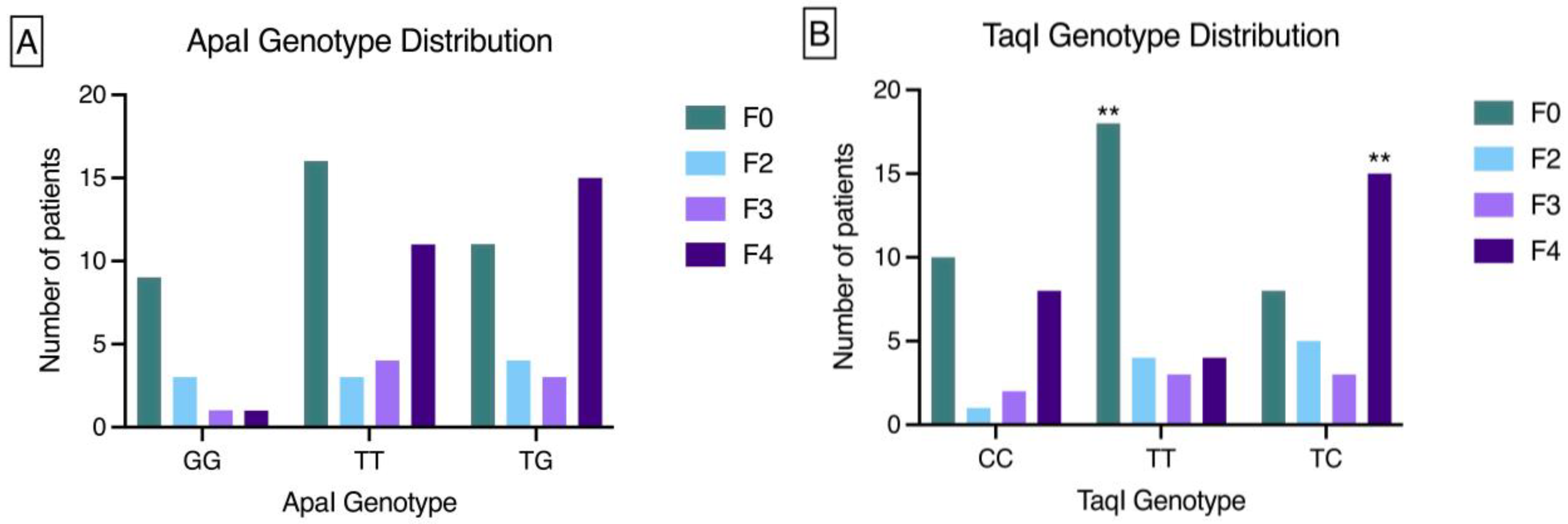
VDR genotype association with fibrosis stage
Discussion
Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Programme. 2017. Global hepatitis report, 2017. Available online: http://apps.who.int/iris/bitstream/10665/255016/1/9 789241565455-eng.pdf?ua=1 (accessed on 15 May 2019).
- Bartenschlager, R.; Baumert, T.F.; Bukh, J.; et al. Critical challenges and emerging opportunities in hepatitis C virus research in an era of potent antiviral therapy: Considerations for scientists and funding agencies. Virus Res. 2018, 248, 53–62. [Google Scholar] [CrossRef]
- Aleman, S. The hurdle with remaining risk for hepatocellular carcinoma in cirrhotic patients after a hepatitis C cure. Hepatol Med Policy 2016, 1, 11. [Google Scholar] [CrossRef]
- Rüeger, S.; Bochud, P.Y.; Dufour, J.F.; et al. Impact of common risk factors of fibrosis progression in chronic hepatitis C. Gut. 2015, 64, 1605–1615. [Google Scholar] [CrossRef]
- Baur, K.; Mertens, J.C.; Schmitt, J.; et al. Combined effect of 25-OH vitamin D plasma levels and genetic vitamin D receptor (NR 1I1) variants on fibrosis progression rate in HCV patients. Liver Int. 2012, 32, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Scalioni, L.P.; Santos, B.R.D.; Spritzer, P.M.; et al. Impact of vitamin D receptor and binding protein gene polymorphisms in clinical and laboratory data of HCV patients: Cross sectional study. Medicine 2018, 97, e9881. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; et al. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J Bone Miner Res. 1998, 13, 325–349. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-A review of recent evidence. Autoimmun Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Ertan, T.; et al. Association between vitamin D receptor gene polymorphism and Alzheimer’s disease. Tohoku J Exp Med. 2007, 212, 275–282. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet. 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Morrison, N.A.; Qi, J.C.; Tokita, A.; et al. Prediction of bone density from vitamin D receptor alleles. Nature 1994, 367, 284–287. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Kalafateli, M.; et al. Prognostic significance of vitamin D receptor (VDR) gene polymorphisms in liver cirrhosis. Sci Rep. 2018, 8, 14065. [Google Scholar] [CrossRef]
- Rau, M.; Baur, K.; Geier, A. Host genetic variants in the pathogenesis of hepatitis C. Viruses 2012, 4, 3281–3302. [Google Scholar] [CrossRef]
- El-Edel, R.; Mostafa, M.; Montaser, B.; El-Hag Ali, Y. Study of Apa-I vitamin D receptor gene polymorphism in patients with hepatocellular carcinoma. Menoufia Med J. 2017, 30, 619. [Google Scholar] [CrossRef]
- Hung, C.H.; Chiu, Y.C.; Hu, T.H.; et al. Significance of vitamin D receptor gene polymorphisms for risk of hepatocellular carcinoma in chronic hepatitis C. Transl Oncol. 2014, 7, 503–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, N.; Yu, R.T.; Subramaniam, N.; et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013, 153, 601–613. [Google Scholar] [CrossRef] [PubMed]
- de Lédinghen, V.; Vergniol, J. Transient elastography (FibroScan). Gastroenterol Clin Biol. 2008, 32, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Omar, N.M.; Mohammed, S.A.; Deiab, A.G. The significance of vitamin D receptor gene polymorphisms for susceptibility to hepatocellular carcinoma in subjects infected with hepatitis C virus. Gastroenterol Hepatol. 2017, 7, 00246. [Google Scholar] [CrossRef][Green Version]
- Cusato, J.; Boglione, L.; De Nicolò, A.; et al. Vitamin D pathway gene polymorphisms and hepatocellular carcinoma in chronic hepatitis C-affected patients treated with new drugs. Cancer Chemother Pharmacol. 2018, 81, 615–620. [Google Scholar] [CrossRef]
- Thanapirom, K.; Suksawatamnuay, S.; Sukeepaisarnjaroen, W.; et al. Genetic associations of vitamin D receptor polymorphisms with advanced liver fibrosis and response to pegylated interferon-based therapy in chronic hepatitis C. PeerJ 2019, 7, e7666. [Google Scholar] [CrossRef]
- Raafat Rowida, I.; Eshra, K.; El-Sharaby, R.; et al. Apa1 (rs7975232) SNP in the vitamin D receptor is linked to hepatocellular carcinoma in hepatitis C virus cirrhosis. Br J Biomed Sci. 2020, 77, 53–57. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Jittikoon, J. Vitamin D and liver fibrosis: Molecular mechanisms and clinical studies. Biomed Pharmacother. 2019, 109, 1351–1360. [Google Scholar] [CrossRef]
- Agliardi, C.; Guerini, F.R.; Saresella, M.; et al. Vitamin D receptor (VDR) gene SNPs influence VDR expression and modulate protection from multiple sclerosis in HLA-DRB1*15-positive individuals. Brain Behav Immun. 2011, 25, 1460–1467. [Google Scholar] [CrossRef]
- Gisbert-Ferrándiz, L.; Salvador, P.; Ortiz-Masiá, D.; et al. A single nucleotide polymorphism in the vitamin D receptor gene is associated with decreased levels of the protein and a penetrating pattern in Crohn’s disease. Inflamm Bowel Dis. 2018, 24, 1462–1470. [Google Scholar] [CrossRef]
- Ding, N.; Downes, M.; Liddle, C.; Evans, R.M.; Ding, N.; Downes, M.; et al. Vitamin D receptor/SMAD genomic circuit gates fibrotic response. US20160106762A1, 2016. Available online: https://patents.google.com/patent/US20160106762/en?oq=VITAMINDRECEPTOR%2fSMADGENOMIC+C IRCUIT+GATES+FIBROTC+RESPONSE (accessed on 16 May 2019).



 |
© GERMS 2022.
Share and Cite
Beka, A.A.; Papadopoulos, V.; Mylopoulou, T.; Panopoulou, M.; Karakasiliotis, I.; Mavromara, P.; Mimidis, K.; Veletza, S. Association Between Vitamin D Receptor Gene Polymorphisms and Fibrosis Susceptibility in Greek Patients with HCV Infection. Germs 2022, 12, 384-393. https://doi.org/10.18683/germs.2022.1342
Beka AA, Papadopoulos V, Mylopoulou T, Panopoulou M, Karakasiliotis I, Mavromara P, Mimidis K, Veletza S. Association Between Vitamin D Receptor Gene Polymorphisms and Fibrosis Susceptibility in Greek Patients with HCV Infection. Germs. 2022; 12(3):384-393. https://doi.org/10.18683/germs.2022.1342
Chicago/Turabian StyleBeka, Angeliki Anna, Vasileios Papadopoulos, Theodora Mylopoulou, Maria Panopoulou, Ioannis Karakasiliotis, Penelope Mavromara, Konstantinos Mimidis, and Stavroula Veletza. 2022. "Association Between Vitamin D Receptor Gene Polymorphisms and Fibrosis Susceptibility in Greek Patients with HCV Infection" Germs 12, no. 3: 384-393. https://doi.org/10.18683/germs.2022.1342
APA StyleBeka, A. A., Papadopoulos, V., Mylopoulou, T., Panopoulou, M., Karakasiliotis, I., Mavromara, P., Mimidis, K., & Veletza, S. (2022). Association Between Vitamin D Receptor Gene Polymorphisms and Fibrosis Susceptibility in Greek Patients with HCV Infection. Germs, 12(3), 384-393. https://doi.org/10.18683/germs.2022.1342




