Abstract
SARS-CoV-2 infection, in the vast majority, affects adult patients. The severity of COVID-19 and mortality are directly correlated with the increasing age of patients and the number of comorbidities. However, with the further development of the COVID-19 pandemic, severe cases of COVID-19 have been reported in children. About 0.8–1% of sick children require hospitalization in an intensive care unit. The main syndromes that cause disease severity and mortality in children are acute respiratory distress syndrome, multisystem inflammation syndrome and multiple organ failure. The rapid development of severe respiratory failure and hypoxemia in respiratory distress syndrome leads to the use of various methods of respiratory therapy, and in case of their ineffectiveness to extracorporeal membrane oxygenation (ECMO). In our report, we present two clinical cases of successful use of ECMO in children with COVID-19, who developed severe ARDS.
Introduction
Since the beginning of the COVID-19 pandemic caused by SARS-CoV-2, low morbidity has been reported in children [1]. Thus, among pediatric patients, an average of 2.5% of patients diagnosed with SARS-CoV-2 required hospitalization, and 0.8% of children required admission to the intensive care unit (ICU). The mortality in the pediatric population of patients with COVID-19 was <0.1%. Among children and adolescents, the highest percentage of hospitalizations (4.6%) was in patients aged 0-4 years [2]. The main complications of SARS-CoV-2 that cause mortality in children are respiratory distress syndrome, multiorgan failure and multisystem inflammatory syndrome [3].
Since the main target organ in COVID-19 is the lungs, it is the acute respiratory distress syndrome that is the main cause of deterioration in patients and their admission to the ICU. According to an international survey examining the availability of extracorporeal membrane oxygenation (ECMO) in pediatric ICU (PICU) that treated patients with COVID-19, admission (multiple choice question) to the PICU was most frequently due to respiratory failure (n=50, 76.9%), followed by multisystem inflammatory syndrome in children (MIS-C, 27.7%, n=18) and cardiac failure (26.2%, n=17) [4]. The rapid development of severe respiratory failure (RF) and hypoxemia leads to the use of various methods of respiratory therapy, and in case of their ineffectiveness, extracorporeal membrane oxygenation is used as the final possible rescue technology [3,5].
In our report, we present two clinical cases of successful use of ECMO in children with COVID-19, who developed severe ARDS and critical hypoxemia, which could not be corrected by traditional methods of respiratory therapy.
Case Reports
- Case 1
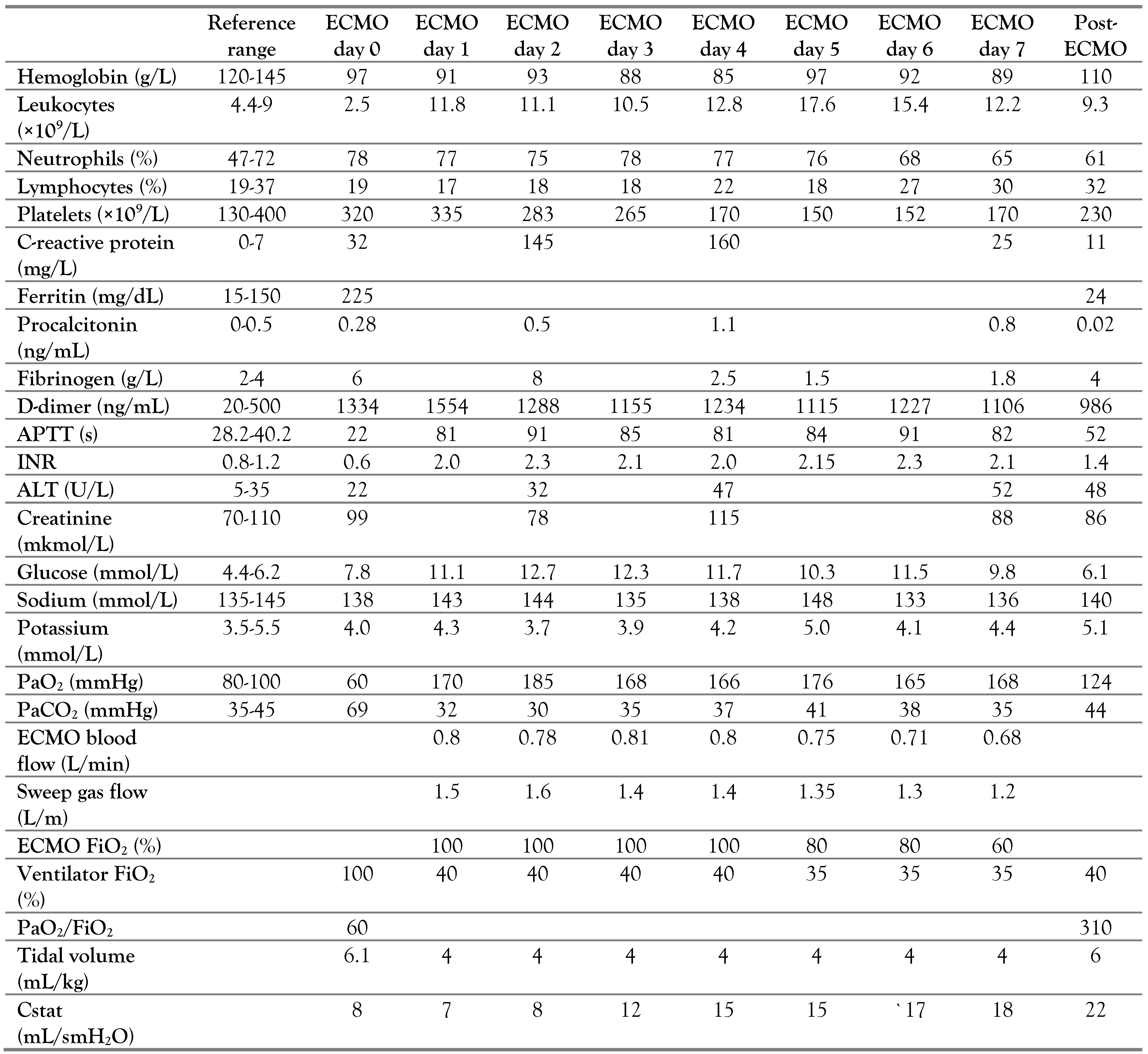
A three-year-old girl (body weight 15 kg) was admitted to the ICU with severe signs of RF. Physical examination revealed shortness of breath, respiratory rate (RR) 72 /min, retraction of intercostal spaces, heart rate (HR) 170/min, sinus tachycardia, SpO2 87%, and body temperature 36.8 °C. Crackles on both sides of the lungs were detected during the auscultation. The chest radiography revealed right-sided focal pneumonia (Figure 1).
Figure 1.
Right-sided focal pneumonia.
The child had been ill for two days, when she developed a low-grade fever.
The next day, the temperature rose to 37.6 °C, there were weakness and coughing. On the third day of the disease there was breathlessness, which progressed rapidly and led to hospitalization.
A week previously, the girl’s father had been diagnosed with COVID-19 confirmed by a positive PCR test for SARS-CoV-2.
Oxygen was administered through facemask (O2 flow at 10 L/min). Ceftriaxone, azithromycin and dexamethasone were administered, and correction of fluid and electrolyte disorders were performed. As the oxygen therapy was not accompanied by a significant improvement in oxygenation (SpO2 90-91%, PaO2 68 mmHg, PaCO2 55 mmHg, pH 7.27), dyspnea with retraction of muscles, after 5 hours non-invasive ventilation was transitioned: NIV CPAP/PSV (FiO260%, PEEP 8 cmH2O, PIP 5 cmH2O). A temporary improvement in oxygenation was achieved: PaO2/FiO2 133, PaO280 mmHg, PaCO2 57 mmHg.
There was a further progression of RF and hypoxemia (PaO2/FiO2 117) on the background of NIV CPAP/PSV in the child, as well as the deterioration of the neurological status of the patient on the Glasgow scale (the decrease in the GCS score from 14 to 12). The control chest X-rays (performed in 24 hours) revealed a deterioration of the radiological imaging (Figure 2).

Figure 2.
The increase of the intensity of opacities on the right side, the appearance of new bilateral infiltrates.
In 25 hours, the patient was administered mechanical ventilation: P/SIMV, FiO2 50%, PIP 12 cmH2O, PEEP 10 cmH2O, TV 6 mL/kg, RR 25/min, I/E 1: 1.5. Given SvO2 63%, the infusion of dobutamine 5 μg/kg/min was prescribed. The dynamics of laboratory parameters is shown in Table 1.

Table 1.
The dynamics of laboratory parameters. ECMO settings and mechanical ventilation. Case 1.
After 36 hours, a positive PCR test for SARS-CoV-2 was received.
For the next 96 hours, the child was on the mechanical ventilation: P/SIMV in prone position, and required myoplegia and fixed parameters of mechanical ventilation: Ppeak 29 cmH2O, PIP15 cmH2O, PEEP 14 cmH2O, TV 6 mL/kg, RR 25/min, I/E 1:1.2. The patient’s condition deteriorated, the patient needed an increase in FiO2 from 60% to 100%. The negative dynamics of PaO2/FiO2 from 117 to 80 was noted. The ultrasound examination of the lungs showed signs of the interstitial syndrome (a large number of B-lines, single A-lines) and a symptom of consolidation in the basal areas of the lungs on both sides. The reduced ejection fraction of the left ventricle (IF 45%) was observed. The child received meropenem, fluconazole, dobutamine 7.5 μg/kg/min, furosemide 7 mg/kg/d, dexamethasone 8 mg/d, heparin 5 IU/kg/h.
On the 5th day after the start of mechanical ventilation, the child was transitioned to high-frequency oscillatory ventilation (HFOV): FiO2 100% Paw 25 cmH2O, ΔP33%, frequency 7Hz. However, no significant improvement in oxygenation was achieved (PaO2/FiO272).
For the next 48 hours, a decrease in oxygenation was observed (PaO2/FiO260). It was decided to transition the child to V-V ECMO (7 days from the beginning of mechanical ventilation). The right internal jugular vein (return cannula: 14 Fr) and the left femoral vein (access cannula: 16 Fr) were cannulated. V-V ECMO settings:—RPM (pump speed) 3125, LPO (blood flow rate) 0.7-0.8, FiO2 100%. Protective mechanical ventilation was performed: P/SIMV, FiO2 40%, PIP 14 cmH2O, PEEP 10 cmH2O, TV 4 mL/kg, RR 15/min, I/E 1:1.5. The child required prolonged analgesia and sedation, heparinization (heparin 10-15 units/kg/h, target values of APTT 80-90 s).
As a result of V-V ECMO, there was an improvement in oxygenation of PaO2 170-180 mmHg, and also respiratory mechanics; static lung compliance (Cstat) increased from 8 mL/cmH2O to 22 mL/cmH2O. The duration of V-V ECMO was 7 days. Subsequently, the convection ventilation continued. P/SIMV: PIP 18 cmH2O, PEEP 10 cmH2O, FiO240%, RR 20/min. Satisfactory oxygenation rates (PaO2/FiO2310) were achieved—Table 1.
After six days, the child was weaned from mechanical ventilation.
The following complications were observed in the course of treatment in the ICU: thrombocytopenia, short-term steroid diabetes and cognitive disorders, hematoma of the anterior abdominal area. Hematoma developed after cannulation of the femoral artery on the background of heparin therapy. The volume of the hematoma was 250-300 mL, it led to intra-abdominal hypertension. The size of the hematoma, its anatomical location was diagnosed by ultrasound examination. The child underwent surgical drainage of the hematoma. After ten days, she was transferred from the ICU.
The child had mild neurological disorders. Thus, we observed a decrease in the strength of the muscles of the lower and upper extremities, impaired articulation and difficulty forming long sentences, as well as fine motor skills such as inability to draw simple pictures, hold a pencil, brush your teeth.
The total duration of treatment in the hospital was 67 days.
- Case 2
A 17-year-old girl (body weight—50 kg) was admitted to the admission department with complaints of dyspnea, a cough, fever 38.5 °C, and general fatigue. Physical examination: RR 24/min, HR 111/min, BP 100/60 mmHg, SpO297%. The patient was hospitalized in the pediatric department. A positive PCR test for SARS-CoV-2 was received.
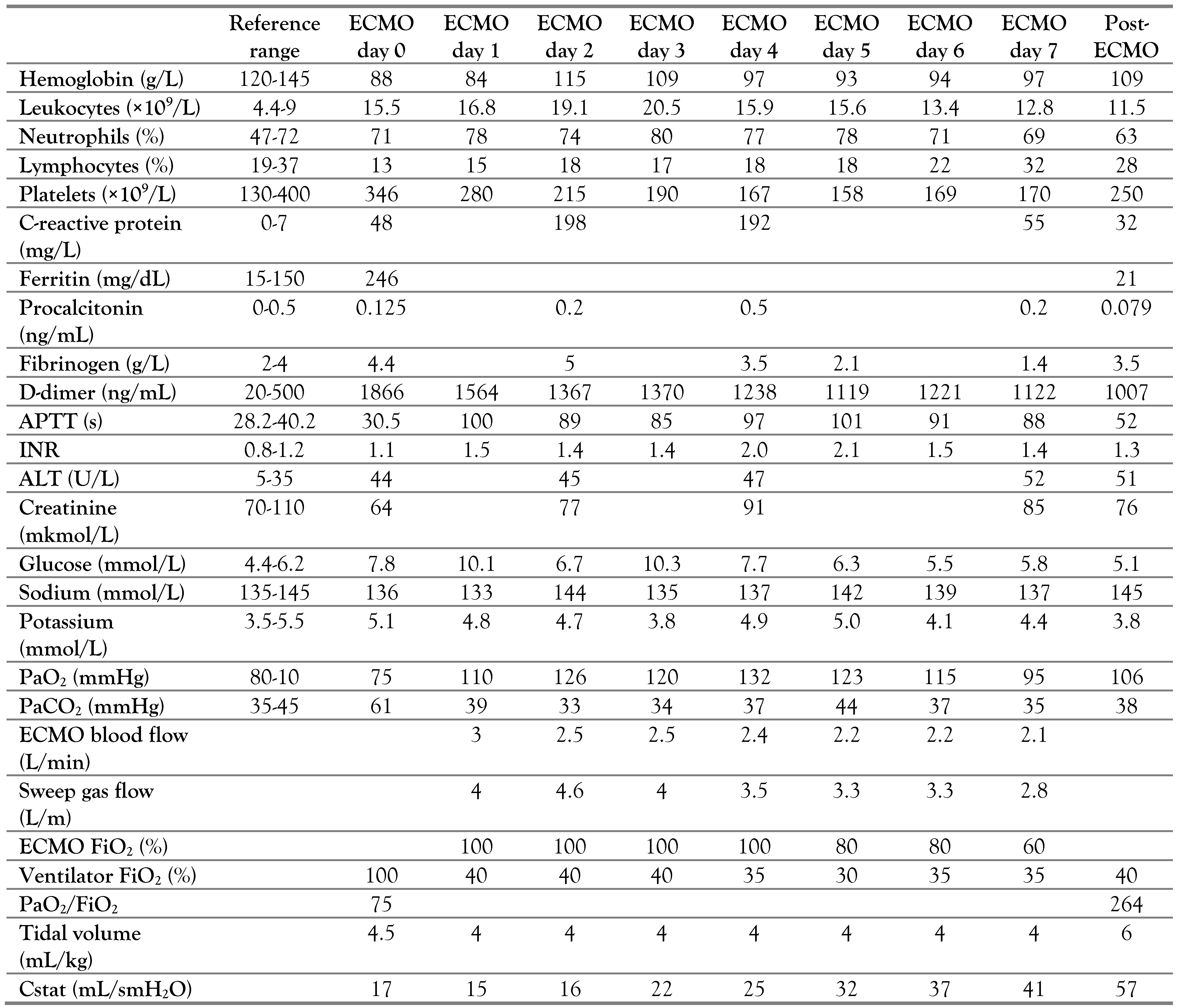
The child’s condition deteriorated within three days. The RF progressed; RR 35/min, SpO286%, oxygen dependence, HR 110/min, BP 100/60 mmHg, the symptom of capillary refill > 3 s, cold extremities. Bilateral polysegmental pneumonia was detected on the chest radiograph (Figure 3). The lung ultrasound showed signs of interstitial syndrome on both sides, alveolar consolidation on the left, left-sided hydrothorax.
Figure 3.
Bilateral polysegmental pneumonia.
The patient was transferred to the ICU. Non-invasive lung ventilation was administered: NIV BiPAP, PIP 12 cmH2O. PEEP 7 cmH2O. FiO2 60%. Acid-base balance: pH 7.4 pCO2 30, PaO2 86, PaO2/FiO2143.
After 16 hours of non-invasive mechanical ventilation, due to the progression of RF (dyspnea 43/min, retraction of respiratory muscles, no improvement PaO2), the child was transitioned to mechanical ventilation: P/SIMV, FiO2 60%, PIP 15 cmH2O, PEEP 10 cmH2O, I/E 1-1.5, TV 320 mL/kg, RR 16/min. The ventilation was performed using prone position as well as myoplegia.
The following drug therapy was administered: remdesivir, cefepime, ciprofloxacin, dexamethasone 6 mg/d, enoxaparin 0.4 mL/d, nutritional therapy (enteral feeding through a tube), and sedation.
On the third day of mechanical ventilation, the girl had a complication—left-sided pneumothorax. The urgent drainage of the pleural cavity (Figure 4) was performed, as well as reduction of mechanical ventilation parameters: P/SIMV, FiO2 80%, PIP 12 cmH2O, PEEP 8 cmH2O, I/E 1-1.5, TV 300 mL/kg.
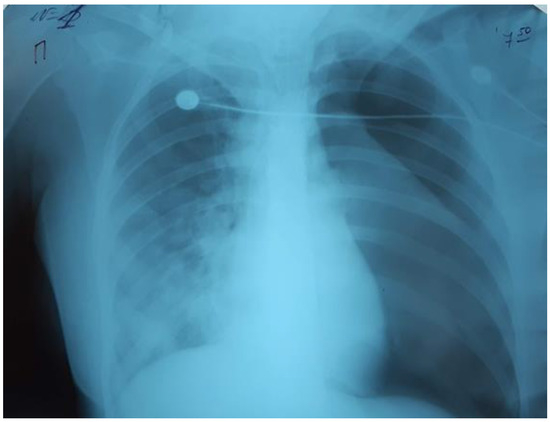
Figure 4.
Left-sided pneumothorax.
Over the next 24 hours, there was a progressive decrease in oxygenation: PaO2/FiO281, PaO2 80 mmHg, PaCO2 61 mmHg. The chest radiograph showed that the left lung was partly collapsed; there was an active air discharge through the drainage. The drainage of the left pleural cavity and the second drainage were performed (Figure 5).
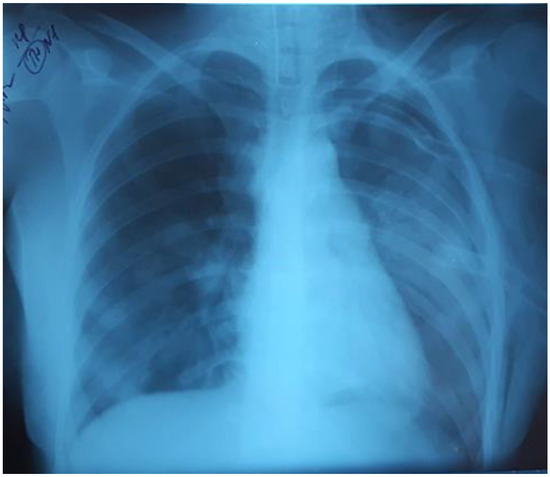
Figure 5.
The left lung was partly collapsed.
There was no positive dynamics within the next 24 hours. The need for ECMO was considered. Ventilation: P/SIMV, FiO2 100%, PIP 11 cmH2O, RR 25 PEEP 8 cmH2O, I/E 1-1.5, TV 240 mL/kg, which led to the deterioration of oxygenation of PaO2/FiO2 74.
On the fifth day from the beginning of mechanical ventilation, there was a further decrease in oxygenation: PaO2/FiO2 75, PaO2 75 mmHg, PaCO261 mmHg, the child received V-V ECMO. The patient was cannulated vena saphena magna dextra (return cannula: №22) and vena saphena magna sinistra (access cannula: №22). Productivity of 2.2–2.5 L/min.
V-V ECMO settings: RPM (pump speed) 3440, LPO (blood flow rate) 2.5-3 L/min, FiO2 100%. The protective mechanical lung ventilation: P/SIMV, FiO2 40%, PIP 12 cmH2O, PEEP 8 cmH2O, TV 300 mL/kg, RR 12 min, I/E 1: 1.5.
The child required prolonged analgesia and sedation and heparinization. The dynamics of laboratory parameters is shown in Table 2.

Table 2.
The dynamics of laboratory parameters. ECMO settings and mechanical ventilation. Case 2.
As a result of the use of ECMO, the oxygenation indicators were improved: PaO2/FiO2264, PaO2 132 mmHg, PaCO2 38 mmHg. The positive dynamics on pulmonary mechanics was also noted: Cstat increased from 17 mL/cm H2O to 57 mL/cmH2O. The duration of ECMO was seven days. Eight days after weaning from ECMO, the child was extubated and performed spontaneous breathing. Two days later, the child was transferred from the ICU to the pediatric ward. After 12 days, the child was discharged in satisfactory condition. The following side effects were observed: thrombocytopenia and transient cognitive impairment. The total duration of hospitalization was thirty-nine days.
Discussion
The ECMO should be considered as an option for rescue therapy in patients when mechanical ventilation, nitric oxide, and HFOV, do not provide adequate blood oxygenation. According to EuroELSO (Extracorporeal Life Support Organization), the frequency of ECMO use during the COVID-19 pandemic was 0.5-1% of all patients hospitalized in the ICU. Although cases of pneumonia caused by SARS-CoV-2 have been reported in infants, children and young adults, these patients generally performed well and rarely required extracorporeal life support [2,5]. According to the EuroELSO survey, which was conducted on January 28, 2021, in European countries, only ten cases of ECMO treatment in children were officially registered, while in adult patients more than a thousand cases of ECMO were registered. As of April 20, 2022, the number of children who received ECMO had increased to 47 children, 17 of whom had ARDS [6].
As for the survival of children, the results of ECMO treatment in the pediatric population are better than in adults, on average 57% [7]. The predictors of mortality in patients who required ECMO are mechanical ventilation that exceeds two weeks before ECMO, multiple organ failure, and comorbid conditions. Therefore, it is important to take a timely decision to start ECMO, before the onset of dysfunction of other vital systems of the body.
According to the recommendations of ELSO (2015), Indications for Pediatric Respiratory Extracorporeal Life Support, “the decision to start ECMO should be based on the negative dynamics of oxygenation before reaching the critical values of PaO2/FiO2” [8]. At the same time, according to the guidelines of the ELSO Coronavirus Disease 2019 Interim Guidelines, if the medical institution is not equipped with ECMO, the decision to transfer to the ECMO center can be made at PaO2/FiO2 <100, without waiting for the PaO2/FiO2 criteria <80 [9]. The purpose of protective lung ventilation is to minimize development of ventilator-associated lung injury (VALI). The main manifestations of VILI include pulmonary barotrauma and biotrauma of the lungs. Biotrauma is associated with oxidative stress and activation of pro-inflammatory cytokines. According to current data, the main factors causing VALI are tidal volume (VT)> 6 mL/kg, excessive inspiratory airway pressure (Pinsp >35 cm H2O), too low or incorrectly set positive end-expiratory pressure (PEEP), and also usage of high concentrations of oxygen (FiO2). After the start of ECMO, we applied protective ventilation to both patients. We reduced the TV to 4 mL/kg, PEEP was selected using the PV loop of graphical monitoring of ventilation. We also tried to keep the driving pressure (plateau pressure minus PEEP) <15 cmH2O. After achieving satisfactory oxygenation thanks to ECMO, we reduced FiO2 to 40% [10,11,12,13].
A team of cardiac surgeons performed vascular cannulation for our patients using such equipment (Cardiohelp, Maquet Cardiopulmonary AG, Germany).
The most common complication developing in children on ECMO is the threatening bleedings that result from anticoagulant therapy. According to H. J. Dalton et al., bleedings occurred in 70.2% of children on ECMO, including intracranial hemorrhage in 16% of patients, which was independently associated with a higher daily risk of death [14]. Our patient developed a large hematoma of the anterior abdominal area, probably due to vessel injury during cannulation. As the result of hematoma, intraabdominal hypertension developed and required surgical drainage.
Cognitive disorders in children have been reported in a review by J. Ju-Ming Wong et al., who state that more than a third of infants who received ECMO had cognitive impairment [15]. In another review, John C Lin reports that 42% of children who were treated with ECMO had cognitive impairment [16].
Conclusions
COVID-19-associated life-threatening conditions are much less common in children compared to adults. Nevertheless, in certain cases where critical hypoxemia cannot be corrected by traditional respiratory support, ECMO may represent a saving technology and it has been observed that subject to its timely use in pediatric patients the treatment was successful in more than half of cases.
Author Contributions
MS and HL were involved in conception, literature review, and writing. NB contributed to data collection and processing. VM contributed to writing, supervision and critical review. All authors read and approved the final version of the manuscript.
Funding
None to declare.
Acknowledgments
The authors express their sincere gratitude to the patients’ parents for the permission to use the data research in writing the article and scientific work. The authors also thank the doctors of the ICU of the Lviv Regional Pediatric Clinical Hospital “OHMATDYT”.
Conflicts of Interest
All authors—none to declare.
Consent
Written informed consent was obtained from the parents for publication of their case report and the accompanying images.
References
- Leidman, E.; Duca, L.M.; Omura, J.D.; Proia, K.; Stephens, J.W.; Sauber-Schatz, E.K. COVID-19 trends among persons aged 0-24 years. MMWR Morb Mortal Wkly Rep. 2021, 70, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Alonso Díaz, C.; López Maestro, M.; Moral Pumarega, M.T.; Flores Antón, B.; Pallás Alonso, C.R. [First case of neonatal infection due to SARS-CoV-2 in Spain]. An Pediatr. 2020, 92, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, M.; Hoskote, A.; Thiruchelvam, T.; et al. Extracorporeal membrane oxygenation in children with coronavirus disease 2019: Preliminary report from the Collaborative European Chapter of the Extracorporeal Life Support Organization Prospective Survey. ASAIO J. 2021, 67, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Ogino, M.T.; Jeong, I.S.; et al. Pediatric intensive care preparedness and ECMO availability in children with COVID-19: An international survey. Perfusion 2021, 36, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.S.M.; Tosoni, A.; Kim, Y.; Kissoon, N.; Murthy, S. Coronavirus disease 2019 in critically ill children: A narrative review of the literature. Pediatr Crit Care Med. 2020, 21, 662–666. [Google Scholar] [CrossRef] [PubMed]
- ExtraCorporeal Life Support (ECLS). Registry Sashboard of ECMO-Supported COVID-19 Patient Data. 2022. Available online: https://www.elso.org/Registry/FullCOVID-19RegistryDashboard.aspx (accessed on 20 April 2022).
- Zabrocki, L.A.; Brogan, T.V.; Statler, K.D.; Poss, W.B.; Rollins, M.D.; Bratton, S.L. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 2011, 39, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Extracorporeal Life Support Organization (ELSO). Indications for Pediatric Respiratory Extracorporeal Life Support. 2015. Available online: https://www.elso.org/Portals/0/Files/ELSO%20guidelines%20paeds%20resp_May2015.pdf (accessed on 1 March 2015).
- Shekar, K.; Badulak, J.; Peek, G.; et al. Extracorporeal Life Support Organization coronavirus disease 2019 interim guidelines: A consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020, 66, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Marley, R.A.; Simon, K. Lung-protective ventilation. Annu Rev Nurs Res. 2017, 35, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Yang, T.; Dinglas, V.D.; et al. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. a prospective cohort study. Am J Respir Crit Care Med. 2015, 191, 177–185. [Google Scholar] [CrossRef]
- Amato, M.B.; Meade, M.O.; Slutsky, A.S.; et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Serpa Neto, A.; Cardoso, S.O.; Manetta, J.A.; et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome. JAMA 2012, 308, 1651–1659. [Google Scholar] [CrossRef]
- Dalton, H.J.; Reeder, R.; Garcia-Filion, P.; et al. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017, 196, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.M.; Cheifetz, I.M.; Lee, J.H. Extracorporeal membrane oxygenation for severe pediatric respiratory failure. J Emerg Crit Care Med. 2017, 1, 11. [Google Scholar] [CrossRef]
- Lin, J.C. Extracorporeal membrane oxygenation for severe pediatric respiratory failure. Respir Care 2017, 62, 732–750. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2025.