Abstract
Introduction: Human coronavirus NL63 (HCoV-NL63) is one of four common human respiratory coronaviruses. It causes lower respiratory tract infections in young children, elderly and immunosuppressed people, which could result in fatal outcomes. In this time of pandemic, we want to highlight the importance of other coronaviruses infection besides SARS-CoV-2, especially in a patient with underlying conditions like acute lymphoblastic leukemia, receiving immunosuppressive therapy that could result in humoral secondary immunodeficiencies. Case report: We present the case of a 44-year-old Colombian man with acute lymphoblastic leukemia who developed HCoV-NL63 pulmonary infection after the first month of treatment with blinatumomab complicated with severe secondary hypogammaglobulinemia. HCoV-NL63 was detected by multiplex PCR, and HCoV-NL63 viral pneumonia was diagnosed. Hypogammaglobulinemia was studied by determining serum immunoglobulins levels and protein electrophoresis. The treatment consisted of supportive therapy and replacement with intravenous immunoglobulins. After therapy, the patient improved his oxygenation, and the infection was resolved in a few days. Conclusions: This case highlights the relevance of other coronaviruses infections besides SARS-CoV-2 in patients receiving immunosuppressive therapy who develop secondary antibody deficiency, and the importance of replacement therapy with intravenous immunoglobulins at early stage of infection with HCoV-NL63.
Introduction
Coronaviruses are positive-strand RNA viruses that cause a variety of diseases in humans and animals including gastroenteritis and respiratory tract illnesses. Human coronaviruses (HCoV) are primarily associated with respiratory tract infections and several types have been described across time. Some, like SARS-CoV and currently SARS-CoV-2, are implicated in highly contagious lower respiratory tract infection, causing severe acute respiratory syndrome (SARS), a life-threatening lung disease in humans [1]. The human coronavirus NL63 (HCoV-NL63) was first described in 2004 from a seven-month-old child with bronchiolitis and conjunctivitis even after the identification of SARS-CoV in 2003 [2]. HCoV-NL63 is present worldwide and causes mostly upper respiratory infection. It can also cause lower respiratory infection like pneumonia, especially in children, elderly and persons who are immunosuppressed [3]. HCoV-NL63 infections have been reported in severely immunosuppressed patients such as those with hematological diseases [4]. Blinatumomab is a bispecific CD19-directed CD3 T cell engager used for treatment of relapsed or refractory acute lymphoblastic leukemia (ALL) [5]. This agent binds to the surface of protein CD19 expressed by B cells and the CD3 epsilon subunit, which is part of the T cell receptor, creating a cytolytic synapse. This binding promotes the apoptosis and lysis of malignant B cells through the releasing of granules from activated cytotoxic T cells [6]. Blinatumomab treatment could result in secondary effects like neurological toxicity, cytokine release syndrome, hypogammaglobulinemia, cytopenia, and infections [7]. Here, we present a case of HCoV-NL63 pulmonary infection in a patient with acute lymphoblastic leukemia treated with blinatumomab, who developed a secondary humoral immunodeficiency with severe hypogammaglobulinemia. Here, we want to emphasize the importance of other coronavirus infections than SARS-CoV-2 in patients who are receiving immunosuppressive therapy and the utility of immunoglobulin replacement therapy.
Case Report
A 44-year-old Colombian man was admitted in a third level Clinic in Cali, Colombia at the end of February 2021 (rainy season). The patient had been diagnosed with acute lymphoblastic leukemia (ALL) two years previously, with history of two relapses. He referred five days of fever with no apparent cause. He had been treated with blinatumomab as a bridging therapy to stem cell transplant (SCT) and had finished the first month of treatment with blinatumomab ten days previously. Physical examination showed generalized paleness with normal vitals. At the time of admission, the patient was conscious and did not present urinary or respiratory symptoms. Pancytopenia was observed (Table 1). Blood transfusion of irradiated red blood cells and platelets was indicated; however, the pancytopenia did not improve, and other blood transfusions were required. Blood cultures were obtained and treatment with meropenem was initiated due to febrile neutropenia. Twenty-four hours after admission, the patient began to present respiratory symptoms, oxygen desaturation up to 60%, occasional cough, tachycardia, and hypotension. Treatment with vancomycin, amikacin and fluconazole was started. Chest X-ray showed increase in cotton wool opacities with commitment of all quadrants associated with bilateral broncho-pneumonic infiltrate in the consolidation process (Figure 1). Treatment for influenza virus (oseltamivir), Pneumocystis jirovecii (trimethoprim/sulfamethoxazole) and atypical bacterial infection (iv clarithromycin) was indicated, and COVID-19 was suspected. PCR and antigen detection in nasopharyngeal aspirate for SARS-CoV-2 were negative. Multiplex molecular detection of microorganisms (FilmArray, Biofire, USA) was requested. The patient continued presenting a rapid deterioration of his general condition with worsening of the respiratory pattern, desaturation, and severe hypoxemia, requiring non-invasive mechanical ventilation (the patient refused intubation). A CT scan of the lungs revealed severe bilateral bronchopneumonia, inflammatory hilar ganglia, and minor pleural effusion (Figure 2). Microbiological tests for bacteria, fungi, and mycobacteria were negative, but FilmArray was positive for coronavirus HCoV-NL63.There was no obvious contact with other people infected with HCoV-NL63. There is no specific treatment for this virus infection, thus supportive treatment was continued. Owing to the treatment with blinatumomab, secondary antibody deficiency was suspected, and humoral immune response was evaluated. Protein electrophoresis and serum immunoglobulins(IgG, IgA and IgM) levels revealed severe hypogammaglobulinemia (Table 1). Replacement therapy with intravenous immunoglobulins (IVIG) at a dose of 400 mg/kg (every 28 days) was indicated. Interestingly, the patient improved oxygenation diminishing supplementary oxygen requirement after only 24 hours from IVIG administration. The clinical evolution was adequate and the respiratory syndrome improved after 72 hours from IVIG administration (second molecular testing was not available). Treatment with blinatumomab was restarted. After the second dose of IVIG, his general health condition was good, and the patient was discharged. Currently the patient shows a good engraftment after 6 months from stem cell transplant with matched sibling donor, but he is still requiring IVIG due to hypogammaglobulinemia persistence (data not shown).

Table 1.
Laboratory test results.
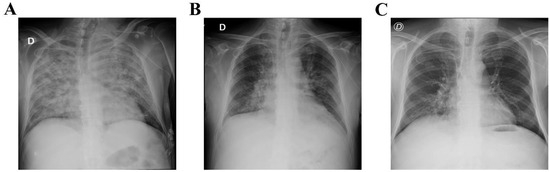
Figure 1.
Chest X-ray before and after intravenous immunoglobulin substitution (IVIG). (A) Chest X-ray with signs of bilateral pneumonia before starting IVIG treatment. Chest X-ray after 3 days (B) and 5 days (C) of IVIG replacement showing improvement of the pneumonia.

Figure 2.
Computed tomography (CT) scan of the chest revealing bilateral bronchopneumonia.
Discussion
Several human coronaviruses have been described, such as HCoV-NL63, HCoV-229E, HCoV-OC43 and HCoV-HKU1. They have low pathogenicity, they are endemic and cause mild diseases [8]. However, they can cause severe lower respiratory tract infection in immunosuppressed patients, and in some cases, they could result in fatal outcomes. Here, we report a case of a 44-year-old Colombian man with ALL who experienced an episode of febrile neutropenia and pneumonia related with a severe HCoV-NL63 infection. There are some reports of human coronavirus infection in patients with hematological disorders. In 2007, Simon et al. reported a case of HCoV-OC43 pneumonia in a 5-year-old boy with ALL and Down syndrome who experienced a febrile neutropenia and pneumonia that did not respond to antibiotic treatment. The diagnosis was made by sequencing the product obtained after RT-PCR for coronaviruses. The condition of this patient improved parallel to an increase of white blood cell counts after several days with neutropenia [9]. In 2016, a fatal outcome of HCoV-NL63 infection in a 27-year-old woman with ALL was reported. This patient developed acute respiratory distress syndrome associated with HCoV-NL63 infection. The virus was detected in bronchoalveolar lavage using real-time RT-PCR, and it was successfully eliminated after treatment with pegylated interferon-alpha (PEG-IFN-α). However, the patient died because of diffuse alveolar hemorrhage triggered by the viral infection [4]. Recently, Chang et al. described an 8-year-old girl with ALL who developed pneumonia with HCoV-OC43 after a febrile neutropenia episode. The diagnosis was made by FilmArray assay confirming the exclusive presence of the HCoV-OC43 virus. This case had a benign course because of rapid improvement of the respiratory status of patient [10]. Li et al. reported a 20-year-old man who after a second allogeneic hematopoietic stem cell transplantation for acute B-lymphocytic leukemia developed complicated pulmonary HCoV-NL63 infection. Diagnosis was made by detection of HCoV-NL63 from pharyngeal swab using metagenomic next-generation sequencing. The patient was treated with antiviral therapy, steroids and immunoglobulins, and his conditions improved satisfactorily [11]. Also, coinfection of alpha-HCoV with SARS-CoV-2 virus have currently been described [3,12]. This emphasizes the importance of other coronaviruses infections in immunosuppressed patients, even in pandemic time, and the continuous circulation of these viruses in the environment.
Interestingly, the HCoV-NL63 pneumonia in our patient was developed after the first month treatment with blinatumomab, and severe hypogammaglobulinemia was also found. Therapy with blinatumomab has been associated with long-term hypogammaglobulinemia [13]. Results of the TOWER study showed the development of hypogammaglobulinemia in 6% of patients treated with blinatumomab compared with 0.9% of those receiving chemotherapy [7]. The immunosuppressive treatment with blinatumomab is currently used for refractory or relapsed B-precursor acute lymphoblastic leukemia. It is a bispecific T-cell engaging (BiTE) antibody that enables the identification of CD19 expressed on B cells by CD3 positive T-cells. This induces a close contact between effector CD3 T-cells and CD19 positive cells with the resultant activation of CD3 T-cells and targeted lysis of CD19 positive cells [6]. CD19 is a B-cell restricted surface antigen expressed in all B-cells (normal and malignant) with the exception of hematopoietic stem cells and differentiated plasma cells. Thus, blinatumomab treatment induces a rapid decrease of peripheral B-cells and depletion of all B-cells including CD19-negative plasma cells because of depletion of its precursors. Therefore, the decrease in immunoglobulin (IgM, IgG, IgA) levels may occur [7].
Serum immunoglobulins have a central role in humoral immune response by binding extracellular pathogens, complement activation and finally pathogen eradication.
Thus, decrease in immunoglobulin levels may be associated with higher risk of infection. It is important to note that infection risks in blinatumomab treatment are not well described. However, higher rates for upper respiratory infection (7.1% vs 0.9%) and serious pneumonia (3.7% vs 0.8%) were observed with blinatumomab versus chemotherapy, respectively [14]. It is recommended to monitor immunoglobulins levels in patients with immunosuppressive therapies and give substitution treatment, especially in cases of patients with a history of serious infections [15]. In this case, replacement therapy with IVIG was indicated until the levels were restored. However, persistent hypogammaglobulinemia was developed, which agrees with the findings of blinatumomab inducing long-term hypogammaglobulinemia recovering only after the regeneration of naive and memory B cells from CD19 negative hematopoietic B cell progenitors [13].
Specific treatment for HCoV-NL63 infection has not yet been described. However, one study suggested the utility of IVIG in the treatment of HCoV-NL63 at early stages of infection. Pyrc et al. demonstrated the potential of neutralizing antibodies in HCoV-NL63 treatment. They showed that the in vitro inhibitory concentration of IVIG was about 10 times lower than the therapeutic dose advised for treatment, suggesting its usefulness in the treatment of this viral infection [16]. Interestingly, in our patient, the infection was quickly resolved after the initiation of IVIG substitution therapy, probably indicating its utility in this viral infection clearance.
Conclusions
This case highlights the infection risk in hematological patients receiving immunosuppressive therapy, side effects of blinatumomab therapy, such as secondary humoral immunodeficiency, and the importance of human coronaviruses infections other than SARS-CoV-2 in pandemic time. It also emphasizes the importance of monitoring immunoglobulin levels in patients receiving immunosuppressive therapies that could result in secondary humoral immunodeficiencies, to provide a timely diagnosis and minimize the risk of future infectious complications.
Author Contributions
CCPA contributed to writing the original draft. LM contributed to review and editing. AFZV contributed to conceptualization, writing-review and editing. All authors read and approved the final version of the manuscript.
Funding
None to declare.
Conflicts of Interest
All authors—none to declare.
Consent
Written informed consent was obtained from the patient for the publication of this case report and the accompanying images.
References
- Hasöksüz, M.; Kiliç, S.; Saraç, F. Coronaviruses and SARS-COV-2. Turk J Med Sci. 2020, 50 (Suppl. 1), 549–556. [Google Scholar] [CrossRef] [PubMed]
- van der Hoek, L.; Pyrc, K.; Jebbink, M.F.; et al. Identification of a new human coronavirus. Nat Med. 2004, 10, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Nadales, A.; Treminio-Quezada, M.; Abad, H.; et al. Critical care management for novel 2019 SARS-CoV-2 and HCoV-NL63 coinfection in a young immunocompromised patient: A Chicago experience. Case Rep Crit Care 2020, 2020, 8877641. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Nellessen, C.; Hahn-Ast, C.; et al. Fatal outcome of human coronavirus NL63 infection despite successful viral elimination by IFN-alpha in a patient with newly diagnosed ALL. Eur J Haematol. 2016, 97, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.J.; Benani, D.J. A review of blinatumomab, a novel immunotherapy. J Oncol Pharm Pract. 2016, 22, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, M.E.; Bargou, R. Blinatumomab: A CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk Lymphoma 2016, 57, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Litzow, M.R. Management of toxicities associated with novel immunotherapy agents in acute lymphoblastic leukemia. Ther Adv Hematol. 2020, 11, 2040620719899897. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Zumla, A.; Locatelli, F.; Ippolito, G.; Kroemer, G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress 2020, 4, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Völz, S.; Fleischhack, G.; et al. Human coronavirus OC43 pneumonia in a pediatric cancer patient with down syndrome and acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2007, 29, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Du, C.J.; Chang, C.C.; et al. Human coronavirus OC43 infection associated pneumonia in a girl with acute lymphoblastic leukemia: A case report. Medicine (Baltimore) 2020, 99, e21520. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, S.; Zheng, Q.; Wu, T. Complicated pulmonary human coronavirus-NL63 infection after a second allogeneic hematopoietic stem cell transplantation for acute B-lymphocytic leukemia: A case report. Medicine (Baltimore) 2021, 100, e26446. [Google Scholar] [CrossRef] [PubMed]
- Chaung, J.; Chan, D.; Pada, S.; Tambyah, P.A. Coinfection with COVID-19 and coronavirus HKU1-the critical need for repeat testing if clinically indicated. J Med Virol. 2020, 92, 1785–1786. [Google Scholar] [CrossRef] [PubMed]
- Zugmaier, G.; Topp, M.S.; Alekar, S.; et al. Long-term follow-up of serum immunoglobulin levels in blinatumomab-treated patients with minimal residual disease-positive B-precursor acute lymphoblastic leukemia. Blood Cancer J. 2014, 4, 244. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, G.; De Greef, J.; Mellinghoff, S.C.; et al. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia 2019, 33, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Pyrc, K.; Bosch, B.J.; Berkhout, B.; et al. Inhibition of human coronavirus NL63 infection at early stages of the replication cycle. Antimicrob Agents Chemother. 2006, 50, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
© GERMS 2025.