Clinical Manifestations, Associated Diseases, Diagnosis, and Treatment of Human Infections Caused by Erysipelothrix rhusiopathiae: A Systematic Review
Abstract
Introduction
Methods
Search strategy
Inclusion and exclusion criteria
Data collection and analysis
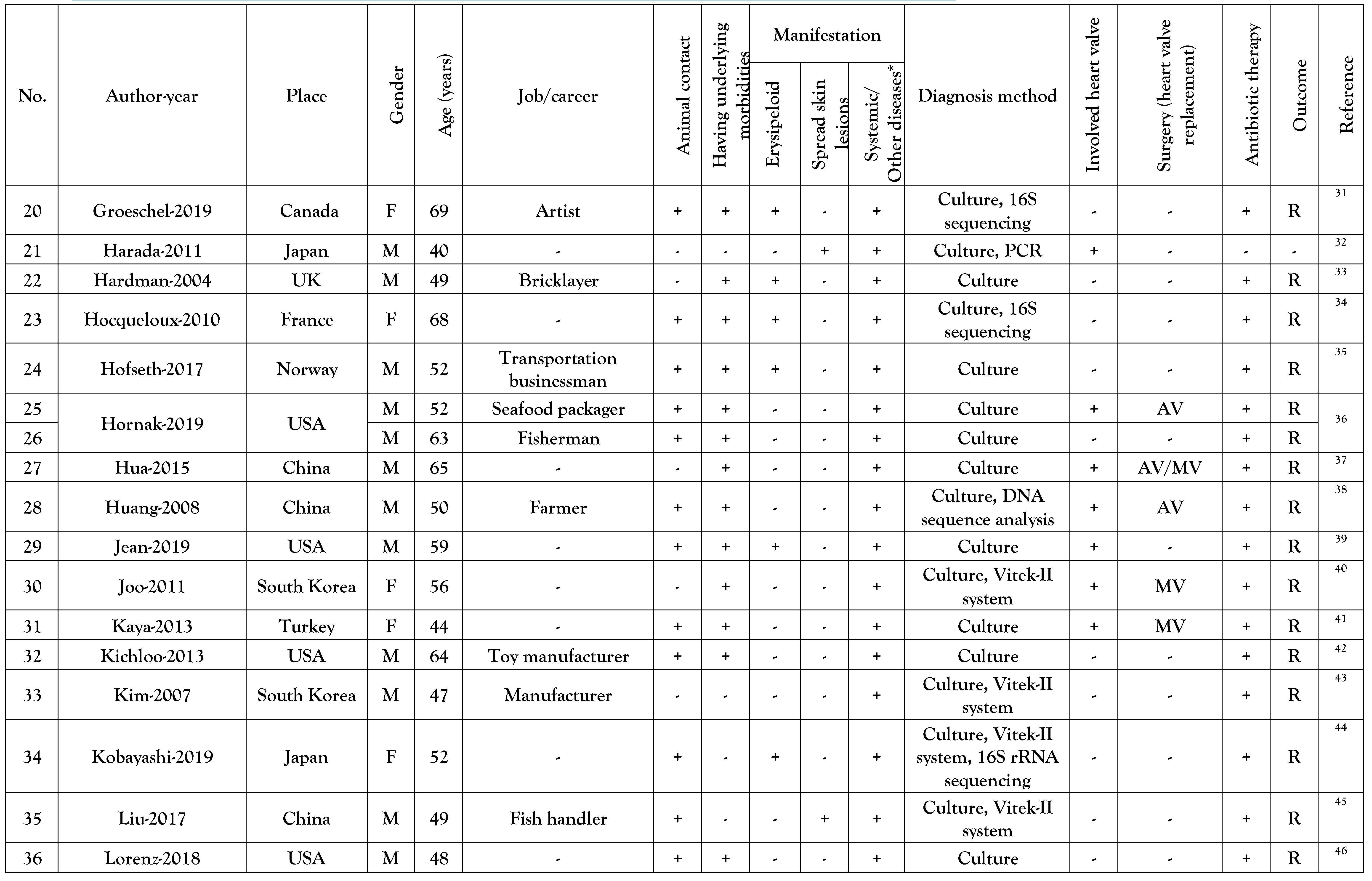
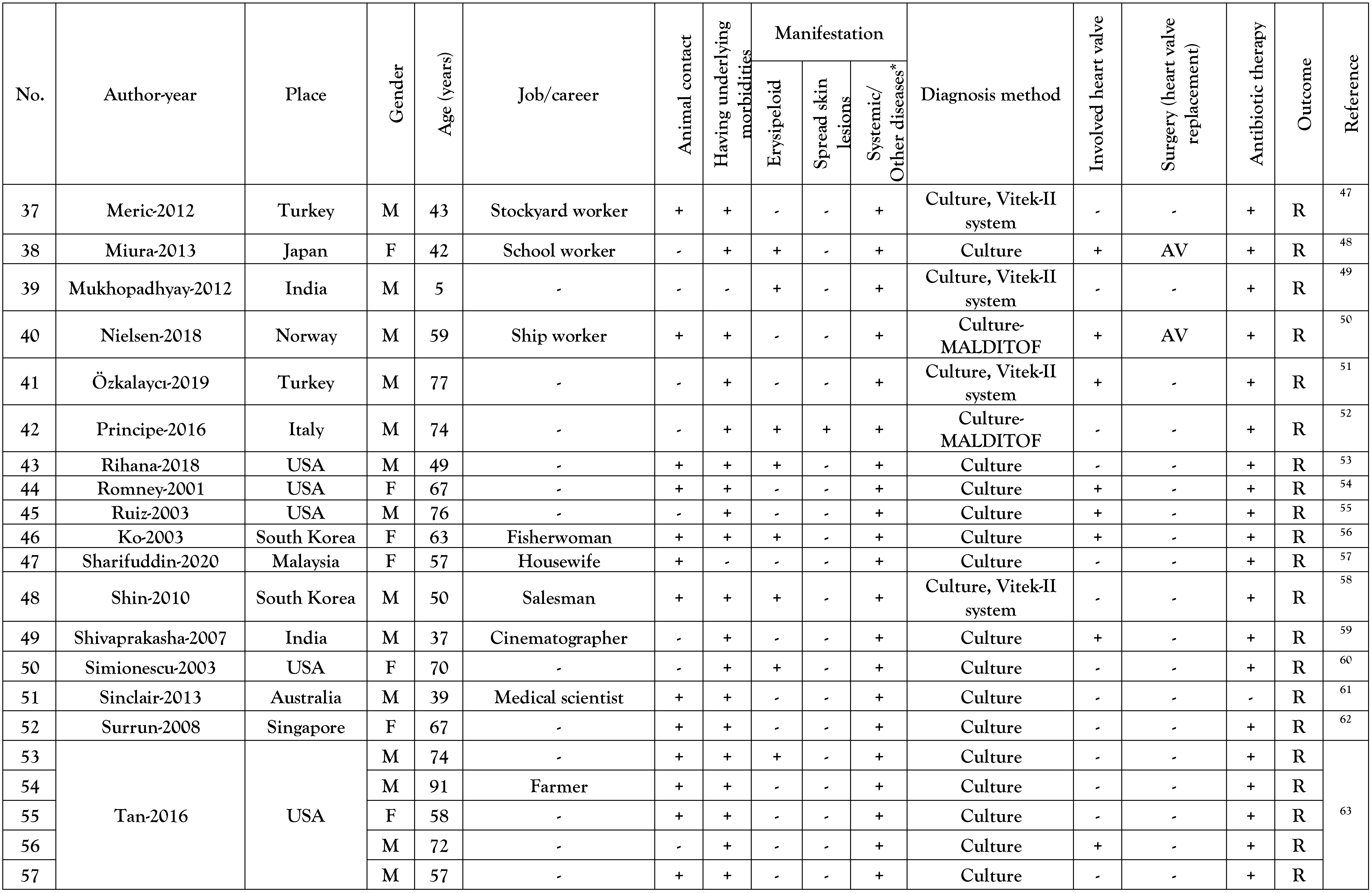
Results
Literature search
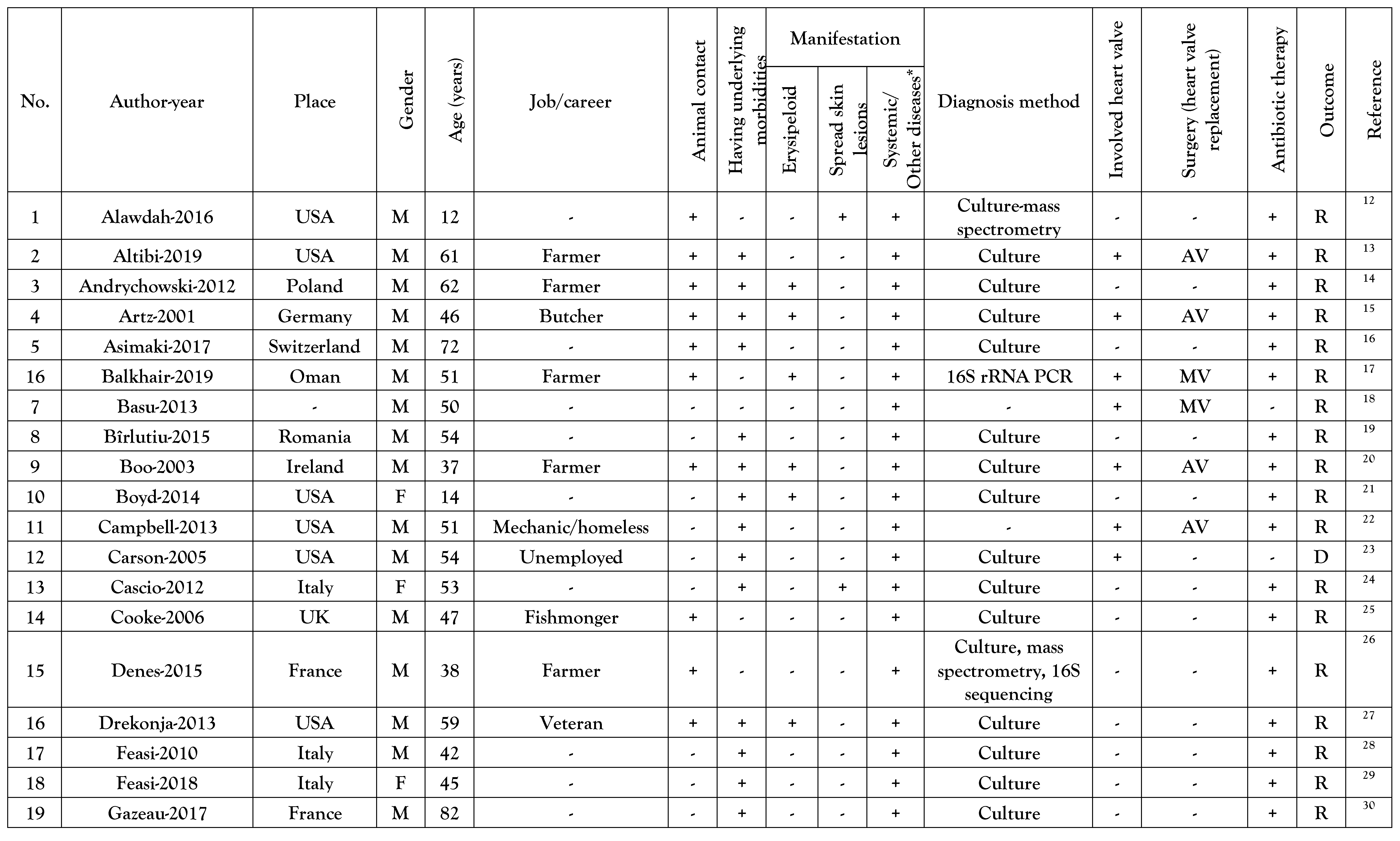
Reported cases general data
Reported cases over years
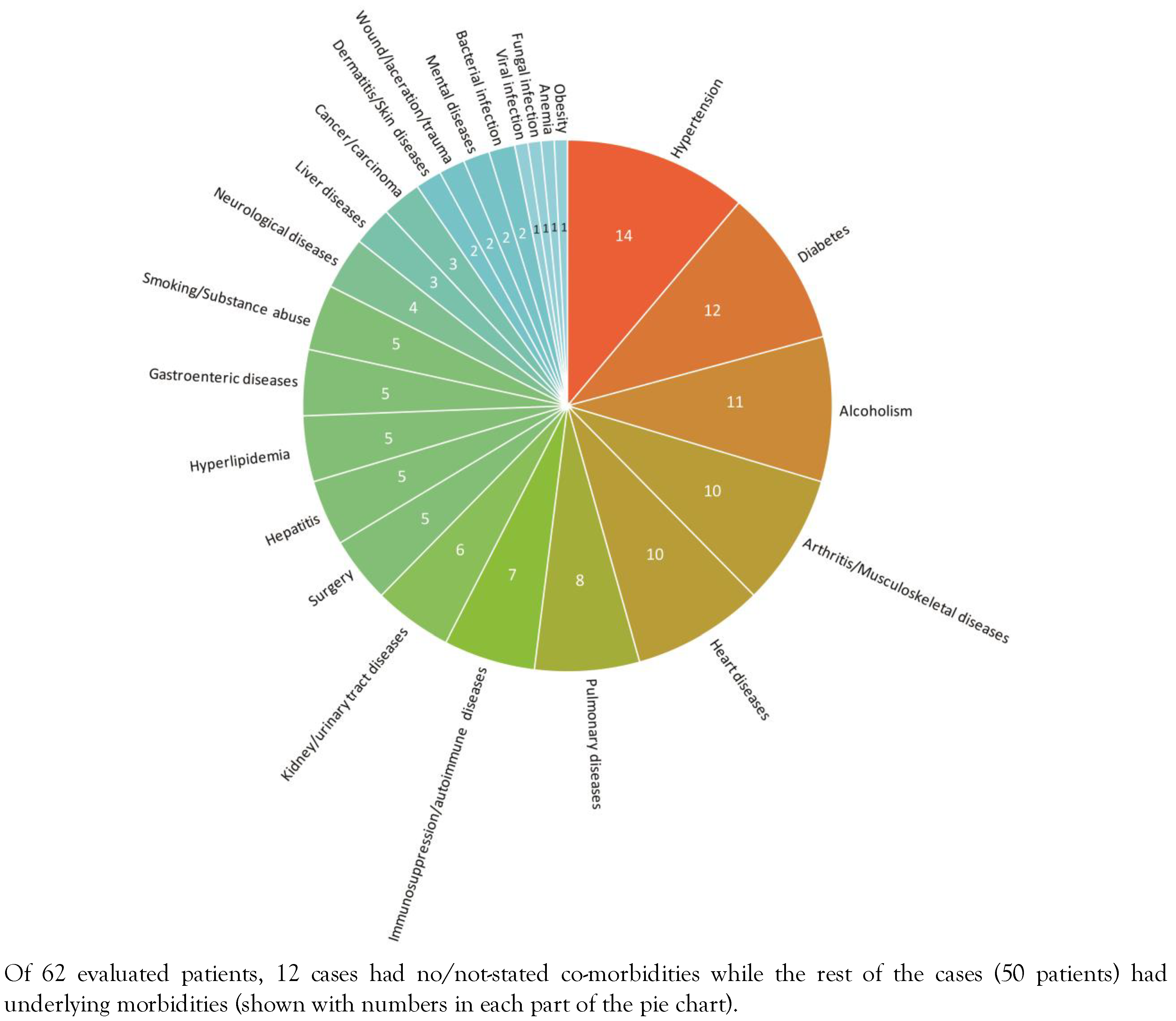
Underlying diseases of the reported cases
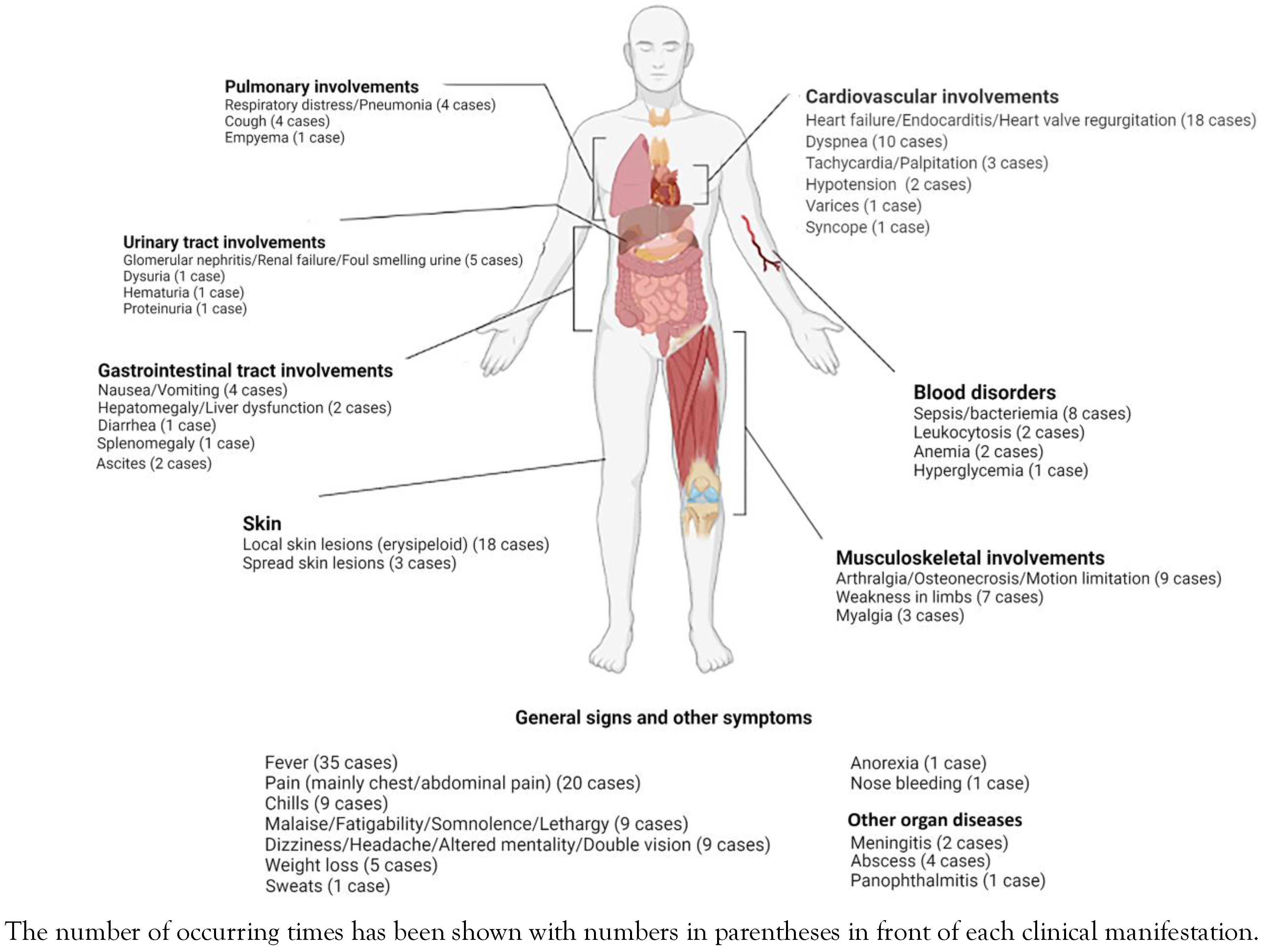
Manifestations of E. rhusiopathiae infections
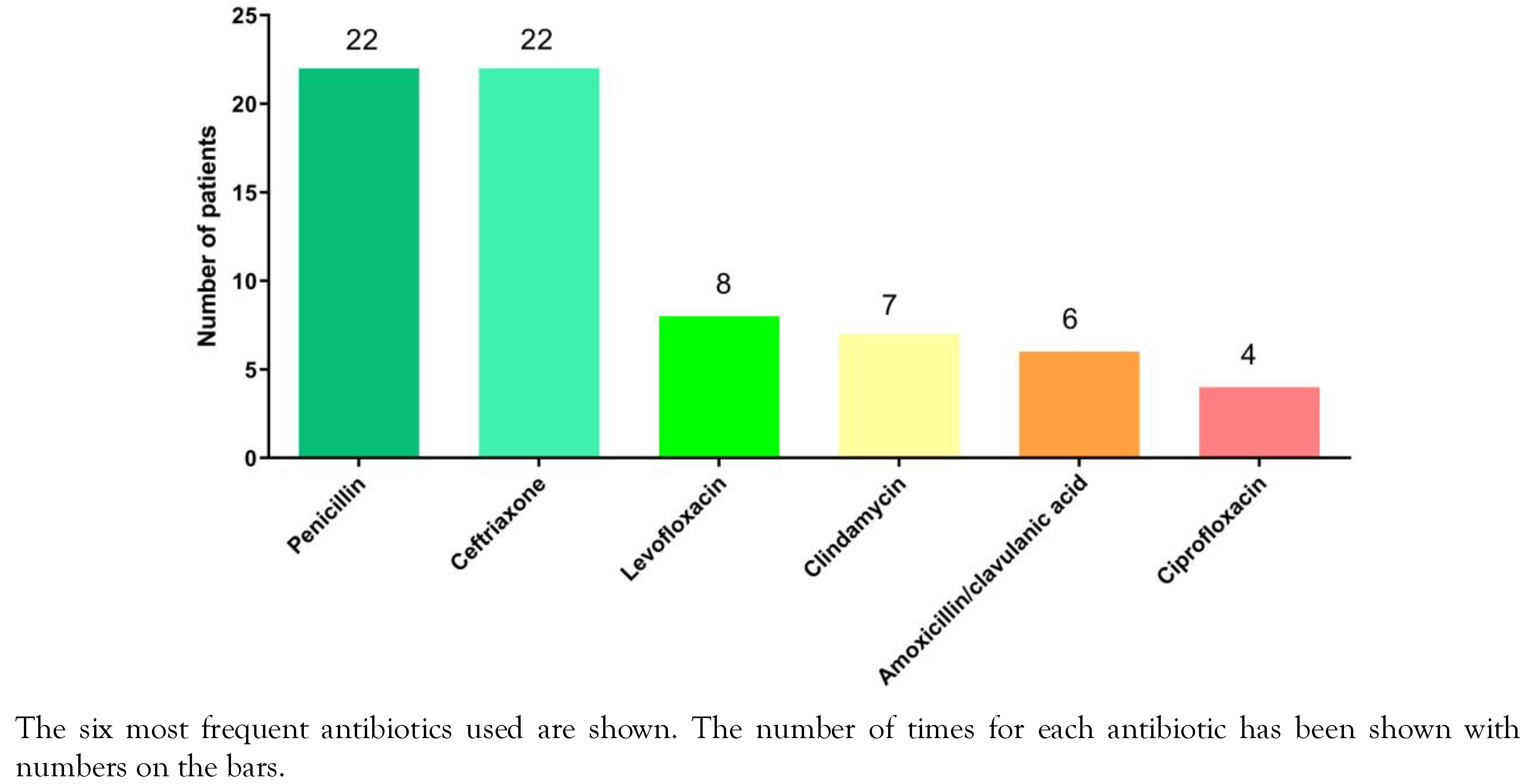
Detection of E. rhusiopathiae infections and antibiotics used
Discussion
Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of interest
References
- Brooke, C.J.; Riley, T.V. Erysipelothrix rhusiopathiae: Bacteriology, epidemiology and clinical manifestations of an occupational pathogen. J Med Microbiol 1999, 48, 789–799. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Y.; Peng, Z.; et al. Comparative genome analysis of a pathogenic Erysipelothrix rhusiopathiae isolate WH13013 from pig reveals potential genes involve in bacterial adaptions and pathogenesis. Vet Sci 2020, 7, 74. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, B.J.; Riley, T.V. Erysipelothrix rhusiopathiae. Vet Microbiol 2010, 140, 405–417. [Google Scholar] [CrossRef]
- Mavrot, F.; Orsel, K.; Hutchins, W.; et al. Novel insights into serodiagnosis and epidemiology of Erysipelothrix rhusiopathiae, a newly recognized pathogen in muskoxen (Ovibos moschatus). PLoS ONE. 2020, 15, e0231724. [Google Scholar] [CrossRef]
- Reboli, A.C.; Farrar, W.E. Erysipelothrix rhusiopathiae: An occupational pathogen. Clin Microbiol Rev. 1989, 2, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, T.A.; Moreno, A.M.; Imada, Y.; Lopez, R.P.; Ferreira Neto, J.S. Characterization of Erysipelothrix rhusiopathiae isolated from Brazilian Tayassu pecari. Trop Anim Health Prod. 2012, 44, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Veraldi, S.; Girgenti, V.; Dassoni, F.; Gianotti, R. Erysipeloid: A review. Clin Exp Dermatol. 2009, 34, 859–862. [Google Scholar] [CrossRef]
- Shimoji, Y. Pathogenicity of Erysipelothrix rhusiopathiae: Virulence factors and protective immunity. Microbes Infect. 2000, 2, 965–972. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, B.J.; Mee, B.J.; Riley, T.V. Neuraminidase production by Erysipelothrix rhusiopathiae. Vet Microbiol. 2005, 107, 265–272. [Google Scholar] [CrossRef]
- 10. Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- World Bank. 2020. Data: World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups%0A (accessed on 3 November 2021).
- Alawdah, L.S.; Campbell, J.N.; Pollock, N.; Watnick, P.I. Erysipelothrix rhusiopathiae suppurative arthritis in a 12-year-old boy after an unusual fresh water exposure. Pediatr Infect Dis J. 2017, 36, 431–433. [Google Scholar] [CrossRef]
- Altibi, A.M.; Khalid, M.; Kak, V.; Patel, B. Native valve endocarditis caused by Erysipelothrix rhusiopathiae: Presenting with refractory heart failure and requiring surgical valve replacement—report on a rare zoonosis. BMJ Case Rep. 2019, 12, e230891. [Google Scholar] [CrossRef]
- Andrychowski, J.; Jasielski, P.; Netczuk, T.; Czernicki, Z. Empyema in spinal canal in thoracic region, abscesses in paravertebral space, spondylitis: In clinical course of zoonosis Erysipelothrix rhusiopathiae. Eur Spine J. 2012, 21 (Suppl. 4), S557–S563. [Google Scholar] [CrossRef]
- Artz, A.; Szabo, S.; Zabel, L.; Hoffmeister, H.M. Aortic valve endocarditis with paravalvular abscesses caused by Erysipelothrix rhusiopathiae. Eur J Clin Microbiol Infect Dis. 2001, 20, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Asimaki, E.; Nolte, O.; Overesch, G.; Strahm, C. A dangerous hobby? Erysipelothrix rhusiopathiae bacteremia most probably acquired from freshwater aquarium fish handling. Infection. 2017, 45, 557–562. [Google Scholar] [CrossRef]
- Balkhair A, Al Lawati H, Al Riyami M, Alameddine T, Al Amin M, Al Adawi B. Erysipelothrix rhusiopathiae endocarditis diagnosed by broad range 16s rRNA PCR gene sequencing. IDCases. 2019, 18, e00584. [CrossRef]
- Basu, R.; Tewari, P. Mitral regurgitation jet around neoannulus: Mitral valve replacement in Erysipelothrix rhusiopathiae endocarditis. Ann Card Anaesth. 2013, 16, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Bîrlutiu, V. Sepsis due to Erysipelothrix rhusiopathiae in a patient with chronic lymphocytic leukemia associated with bronchopneumonia due to Pseudomonas aeruginosa and Escherichia coli: A case report. Can J Infect Dis Med Microbiol. 2015, 26, 108–110. [Google Scholar] [CrossRef]
- Boo, T.W.; Hone, R.; Hurley, J. Erysipelothrix rhusiopathiae endocarditis: A preventable zoonosis? Ir J Med Sci. 2003, 172, 81–82. [Google Scholar] [CrossRef]
- Boyd, A.S.; Ritchie, C.; Fenton, J.S. Cutaneous Erysipelothrix rhusiopathiae (erysipeloid) infection in an immunocompromised child. Pediatr Dermatol. 2014, 31, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Cowan, M. Septicemia and aortic valve endocarditis due to Erysipelothrix rhusiopathiae in a homeless man. Case Rep Infect Dis. 2013, 2013, 923034. [Google Scholar] [CrossRef]
- Carson, H.J.; Perkins, J.T. Visceral botryomycosis in a case of Erysipelothrix rhusiopathiae endocarditis. Hum Pathol. 2005, 36, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Stassi, G.; Cacciola, I.; Saitta, C.; Squadrito, G. Fever and rhomboid target lesion in decompensated cirrhosis. Lancet Infect Dis. 2012, 12, 576. [Google Scholar] [CrossRef]
- Cooke, L.J.; Bowles, K.M.; Craig, J.I.; Sule, O. Occupational injury in a fishmonger with a macular rash, hepatosplenomegaly and pancytopenia. J Clin Pathol. 2006, 59, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Denes, E.; Camilleri, Y.; Fiorenza, F.; Martin, C. First case of osteomyelitis due to Erysipelothrix rhusiopathiae: Pubic osteomyelitis in a gored farmer. Int J Infect Dis. 2015, 30, e133–e134. [Google Scholar] [CrossRef] [PubMed]
- Drekonja, D.M. Erysipelothrix bacteremia without endocarditis: Rare event or under-reported occurrence? Diagn Microbiol Infect Dis. 2013, 77, 280–281. [Google Scholar] [CrossRef]
- Feasi, M.; Bacigalupo, L.; Cappato, S.; et al. Erysipelothrix rhusiopathiae intra-abdominal abscess. Int J Infect Dis. 2010, 14, e81–e83. [Google Scholar] [CrossRef]
- Feasi, M.; Pontali, E.; Usiglio, D.; Mori, M.; Cassola, G. Erysipelothrix rhusiopathiae septicaemia in systemic lupus erythematosus. Infez Med. 2018, 26, 356–358. [Google Scholar]
- Gazeau, P.; Rezig, S.; Quaesaet, L.; Williams, T.; Tande, D.; Ansart, S. Erysipelothrix rhusiopathiae knee prosthesis infection. Med Mal Infect. 2018, 48, 372–373. [Google Scholar] [CrossRef]
- Groeschel, M.; Forde, T.; Turvey, S.; et al. An unusual case of Erysipelothrix rhusiopathiae prosthetic joint infection from the Canadian Arctic: Whole genome sequencing unable to identify a zoonotic source. BMC Infect Dis. 2019, 19, 282. [Google Scholar] [CrossRef]
- Harada, K.; Amano, K.; Akimoto, S.; et al. Serological and pathogenic characterization of Erysipelothrix rhusiopathiae isolates from two human cases of endocarditis in Japan. New Microbiol. 2011, 34, 409–412. [Google Scholar] [PubMed]
- Hardman, S.C.; Carr, S.J.; Swann, R.A. Peritoneal dialysis-related peritonitis with bacteraemia due to Erysipelothrix rhusiopathiae. Nephrol Dial Transplant. 2004, 19, 1340–1341. [Google Scholar] [CrossRef]
- Hocqueloux, L.; Poisson, D.M.; Sunder, S.; Guilbert, S.; Prazuck, T. Septic arthritis caused by Erysipelothrix rhusiopathiae in a prosthetic knee joint. J Clin Microbiol. 2010, 48, 333–335. [Google Scholar] [CrossRef]
- Hofseth, K.; Dalen, H.; Kibsgaard, L.; Nebb, S.; Kümmel, A.; Mehl, A. Infectious tenosynovitis with bloodstream infection caused by Erysipelothrix rhusiopathiae, a case report on an occupational pathogen. BMC Infect Dis. 2017, 17, 12. [Google Scholar] [CrossRef]
- Hornak, J.P.; McLean, M.R.; Webb, C.M.; Keiser, P.H. A comparison of Erysipelothrix rhusiopathiae bacteremia with and without endocarditis in frequent fish handlers. Infect Dis Clin Pract. 2019, 27, 175–177. [Google Scholar] [CrossRef]
- Hua, P.; Liu, J.; Tao, J.; Liu, J.; Yang, Y.; Yang, S. Erysipelothrix rhusiopathiae-induced aortic valve endocarditis: Case report and literature review. Int J Clin Exp Med. 2015, 8, 730–736. [Google Scholar]
- Huang, H.K.; Tsai, C.S.; Chang, F.Y.; et al. Aortic valve endocarditis with perforation of cusps and severe aortic regurgitation caused by Erysipelothrix rhusiopathiae. J Med Sci. 2008, 28, 165–168. [Google Scholar]
- Jean, S.; Lainhart, W.; Yarbrough, M.L. The brief case: Erysipelothrix bacteremia and endocarditis in a 59-year-old immunocompromised male on chronic high-dose steroids. J Clin Microbiol. 2019, 57, e02031-18. [Google Scholar] [CrossRef]
- Joo, E.J.; Kang, C.I.; Kim, W.S.; et al. Acute meningitis as an initial manifestation of Erysipelothrix rhusiopathiae endocarditis. J Infect Chemother. 2011, 17, 703–705. [Google Scholar] [CrossRef]
- Kaya, S.; Gençalioğlu, E.; Yıldırım, S.S.; Altun, G.; Yılmaz, G.; Köksal, I. Native valve endocarditis caused by Erysipelothrix rhusiopathiae in an immunocompetent individual. J Med Microbiol. 2013, 62 Pt 12, 1911–1913. [Google Scholar] [CrossRef]
- Kichloo, A.A.; Hallac, A.; Mousavi, B.; Hirekhan, O. Nonspecific Erysipelothrix rhusiopathiae bacteremia in a patient with subclinical alcoholic liver disease. Case Rep Infect Dis. 2013, 2013, 474593. [Google Scholar] [CrossRef]
- Kim, S.R.; Kwon, M.J.; Lee, J.H.; Lee, N.Y. Chronic meningitis caused by Erysipelothrix rhusiopathiae. J Med Microbiol. 2007, 56 Pt 10, 1405–1406. [Google Scholar] [CrossRef]
- Kobayashi, K.I.; Kawano, T.; Mizuno, S.; Kubo, K.; Komiya, N.; Otsu, S. Erysipelothrix rhusiopathiae bacteremia following a cat bite. IDCases. 2019, 18, e00631. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, M. Shock caused by multidrug-resistant Erysipelothrix rhusiopathiae bacteremia: A rare case report and literature review. J Infect Dev Ctries. 2017, 11, 508–512. [Google Scholar] [CrossRef]
- Lorenz, M.L.; Bouton, T.C.; Caliendo, A.M. First reported case of vertebral osteomyelitis due to Erysipelothrix rhusiopathiae. IDCases. 2017, 11, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Meric, M.; Keceli Ozcan, S. Erysipelothrix rhusiopathiae pneumonia in an immunocompetent patient. J Med Microbiol. 2012, 61 Pt 3, 450–451. [Google Scholar] [CrossRef]
- Miura, T.; Hashizume, K.; Ariyoshi, T.; et al. Active infective endocarditis due to Erysipelothrix rhusiopathiae: Zoonosis caused by vancomycin-resistant gram-positive rod. Gen Thorac Cardiovasc Surg. 2013, 61, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, C.; Shah, H.; Vandana, K.E.; Munim, F.; Vijayan, S. A child with Erysipelothrix arthritis-beware of the little known. Asian Pac J Trop Biomed. 2012, 2, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.J.; Blomberg, B.; Gaïni, S.; Lundemoen, S. Aortic valve endocarditis with Erysipelothrix rhusiopathiae: A rare zoonosis. Infect Dis Rep. 2018, 10, 47–49. [Google Scholar] [CrossRef]
- Özkalaycı, F.; Aydın, M.; Altay, H.; Kocabaş, U.; Pehlivanoğlu, S. Pericarditis due to an unusual microorganism in an immunocompromised patient. Turk Kardiyol Dern Ars. 2019, 47, 507–511. [Google Scholar]
- Principe, L.; Bracco, S.; Mauri, C.; Tonolo, S.; Pini, B.; Luzzaro, F. Erysipelothrix rhusiopathiae bacteremia without endocarditis: Rapid identification from positive blood culture by MALDI-TOF mass spectrometry. A case report and literature review. Infect Dis Rep. 2016, 8, 6368. [Google Scholar] [CrossRef]
- Rihana, N.; Hemminger, A.; Green, S. Novel case of penicillin resistant E. rhusiopathiae septicemia: Case report with review of the literature. IDCases. 2018, 11, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Romney, M.; Cheung, S.; Montessori, V. Erysipelothrix rhusiopathiae endocarditis and presumed osteomyelitis. Can J Infect Dis. 2001, 12, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.E.; Richards, J.S.; Kerr, G.S.; Kan, V.L. Erysipelothrix rhusiopathiae septic arthritis. Arthritis Rheum. 2003, 48, 1156–1157. [Google Scholar] [CrossRef]
- Ko, S.B.; Kim, D.E.; Kwon, H.M.; Roh, J.K. A case of multiple brain infarctions associated with Erysipelothrix rhusiopathiae endocarditis. Arch Neurol. 2003, 60, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Sharifuddin, A.A.; Buandasan, K. A case of panophthalmitis with orbital cellulitis related to Erysipelothrix rhusiopathiae infection. Malays J Ophthalmol. 2020, 2, 48–54. [Google Scholar] [CrossRef]
- Shin, S.J.; Gwak, W.G. Erysipelothrix rhusiopathiae peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis. J Korean Med Sci. 2010, 25, 1234–1236. [Google Scholar] [CrossRef]
- Shivaprakasha, S.; Radhakrishnan, K.; Panikar, D.; Natarajan, K.U.; Shamsul Karim, P.M. Cerebral artery mycotic aneurysm associated with Erysipelothrix rhusiopathiae endocarditis. Infect Dis Clin Pract. 2007, 15, 400–402. [Google Scholar] [CrossRef]
- Simionescu, R.; Grover, S.; Shekar, R.; West, B.C. Necrotizing fasciitis caused by Erysipelothrix rhusiopathiae. South Med J. 2003, 96, 937–939. [Google Scholar] [CrossRef]
- Sinclair, M.; Hawkins, A.; Testro, A. Something fishy: An unusual Erysipelothrix rhusiopathiae infection in an immunocompromised individual. BMJ Case Rep. 2013, 2013, bcr2013008873. [Google Scholar] [CrossRef]
- Surrun, S.K.; Jaufeerally, F.R.; Sim, H.C. Erysipelothrix rhuseopathiae septicaemia with prolonged hypotension: A case report. Ann Acad Med Singap. 2008, 37, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Marcelin, J.R.; Adeel, N.; Lewis, R.J.; Enzler, M.J.; Tosh, P.K. Erysipelothrix rhusiopathiae bloodstream infection—a 22-year experience at Mayo Clinic, Minnesota. Zoonoses Public Health. 2017, 64, e65–72. [Google Scholar] [CrossRef]
- Traer, E.A.; Williams, M.R.; Keenan, J.N. Erysipelothrix rhusiopathiae infection of a total knee arthroplasty. J Arthroplasty. 2008, 23, 609–611. [Google Scholar] [CrossRef]
- Upapan, P.; Chayakulkeeree, M. Erysipelothrix rhusiopathiae bacteremia without endocarditis associated with psoas abscess: The first case report in Thailand. J Med Assoc Thai. 2014, 97 (Suppl. 3), S232–S236. [Google Scholar]
- Allianatos, P.G.; Tilentzoglou, A.C.; Koutsoukou, A.D. Septic arthritis caused by Erysipelothrix rhusiopathiae infection after arthroscopically assisted anterior cruciate ligament reconstruction. Arthroscopy. 2003, 19, E26. [Google Scholar] [CrossRef]
- Volard, B.; Mignot, L.; Piednoir, E.; de Champs, C.; Limelette, A.; Guillard, T. Systemic Erysipelothrix rhusiopathiae infection not associated with endocarditis highlighting bacteriological diagnosis difficulties Case report and literature review. Ann Biol Clin (Paris). 2016, 74, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Shioshita, K.; Takazono, T.; et al. An autopsy case of Erysipelothrix rhusiopathiae endocarditis. Intern Med. 2008, 47, 1437–1440. [Google Scholar] [CrossRef] [PubMed]



    |
© GERMS 2022.
Share and Cite
Rostamian, M.; Rahmati, D.; Akya, A. Clinical Manifestations, Associated Diseases, Diagnosis, and Treatment of Human Infections Caused by Erysipelothrix rhusiopathiae: A Systematic Review. GERMS 2022, 12, 16-31. https://doi.org/10.18683/germs.2022.1303
Rostamian M, Rahmati D, Akya A. Clinical Manifestations, Associated Diseases, Diagnosis, and Treatment of Human Infections Caused by Erysipelothrix rhusiopathiae: A Systematic Review. GERMS. 2022; 12(1):16-31. https://doi.org/10.18683/germs.2022.1303
Chicago/Turabian StyleRostamian, Mosayeb, Donya Rahmati, and Alisha Akya. 2022. "Clinical Manifestations, Associated Diseases, Diagnosis, and Treatment of Human Infections Caused by Erysipelothrix rhusiopathiae: A Systematic Review" GERMS 12, no. 1: 16-31. https://doi.org/10.18683/germs.2022.1303
APA StyleRostamian, M., Rahmati, D., & Akya, A. (2022). Clinical Manifestations, Associated Diseases, Diagnosis, and Treatment of Human Infections Caused by Erysipelothrix rhusiopathiae: A Systematic Review. GERMS, 12(1), 16-31. https://doi.org/10.18683/germs.2022.1303




